
Review Article
J Bacteriol Mycol. 2024; 11(1): 1216.
Listeria monocytogenes in Livestock and Derived Food-Products: Insights from Antibiotic-Resistant Prevalence and Genomic Analysis
Adriana Silva1; Vanessa Silva1-4; Luis M Cintas5; José Eduardo Pereira6,7; Luís Maltez6,7; MD Tanvir Rahman8; Gilberto Igrejas2-4; Patrícia Valentão9; Virgílio Falco10; Patrícia Poeta1,2,6,7*
1Microbiology and Antibiotic Resistance Team (MicroART), Department of Veterinary Sciences, University of Tras- os-Montes and Alto Douro (UTAD), Portugal
2Department of Genetics and Biotechnology, University of Tras-os-Montes and Alto Douro (UTAD), Portugal
3Functional Genomics and Proteomics Unit, University of Tras-os-Montes and Alto Douro (UTAD), Portugal
4Associated Laboratory for Green Chemistry (LAQV-REQUIMTE), University NOVA of Lisboa, Portugal
5Grupo de Seguridad y Calidad de los Alimentos por Bacterias Lácticas, Bacteriocinas y Probióticos (SEGABALBP), Sección Departamental de Nutrición y Ciencia de los Alimentos, Facultad de Veterinaria, Universidad Complutense de Madrid, Spain
6Veterinary and Animal Research Centre (CECAV), University of Tras-os-Montes and Alto Douro (UTAD), Portugal
7Associate Laboratory for Animal and Veterinary Sciences (AL4AnimalS), Portugal
8Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agriculture University, Bangladesh
9REQUIMTE/LAQV, Laborato rio de Farmacognosia, Departamento de Quimica, Faculdade de Farmacia, Universidade do Porto, Portugal
10Chemistry Research Centre (CQ-VR), University of Tras-os-Montes and Alto Douro (UTAD), Portugal
*Corresponding author: Patrícia Poeta Microbiology and Antibiotic Resistance Team (MicroART), Department of Veterinary Sciences, University of Tras- os-Montes and Alto Douro (UTAD), 5000–801 Vila Real, Portugal. Email: ppoeta@utad.pt
Received: February 20, 2024 Accepted: March 20, 2024 Published: March 27, 2024
Abstract
Antibiotics play an important role in veterinary medicine and serve as important tools to maintain animal health and ensure food safety. However, heavy use of antibiotics in animal production can lead to increased antimicrobial resistance from livestock to humans. Foodborne pathogens are a major public health and food safety problem. Listeria monocytogenes cause severe diseases and outbreaks associated to the consumption of contaminated food products, in humans. In the treatment of infections, L. monocytogenes are susceptible to several antimicrobial agents, however, several recent studies have already reported cases of strains resistant to several classes of antibiotics, such as ampicillin, cefotaxime, tetracyclines, sulfonamides, β-lactams, and penicillin among livestock animals, but also the emergence of multi-resistant strains in these environments have also been described in several recent studies. This review focuses on the occurrence and prevalence L. monocytogenes in livestock and derived food-products and strives to provide information on prevalence of L. monocytogenes in livestock animals, and derived food products, and describe the main antimicrobial resistance and genomic analysis in strains associated and isolated from regions worldwide.
Keywords: Antibiotic resistance; Foodborne pathogens; L. monocytogenes; Livestock; Derived food-products
Introduction
Listeria spp. is a non-spore forming, small Gram-positive rod-shaped bacteria belonging to the phylum Firmicutes, class Bacilli, order Bacillales, family Listeriaceae. They are facultatively anaerobe microorganisms, [1-3] and are actively motile, capable of prospering at low temperatures and in severe conditions [4]. They can tolerate salt conditions (NaCl) up to 20% [w/v], grow in a pH range of 4.4-9.6, and thrive in various extreme environmental conditions [1,2] and different environmental niches such as humans, farms, animals, food, food-processing environments, plants, soils, water, silage and sewage [5]. They can survive in water environments and exhibit optimal growth at values around 0.97. They can persist for extended periods at even lower values. such as 0.83 [2]. To distinguish the Listeria spp. a variety of tests need to be carried out, including hemolysis, mannitol with acid production, D-xylose, L-rhamnose, and alpha-methyl-D-mannoside [2].
Listeria is a genus of 28 species of ubiquitous bacteria in different niches [1]. It is grouped into two groups: “Listeria sensu lato” and “Listeria sensu stricto”. “Listeria sensu stricto “includes L. monocytogenes, L. innocua, L. seelgerii, L. welshimeri, and L. marthii. These species are catalase-positive, motile at 30°C, and grow below or at 4°C. “Listeria sensu lato” includes L. grayi, L. fleischmannii, L. floridensis, L. aquatica, L. newyorkensis, L. cornellensis, L. rocourtiae, L. weihenstephanensis, L. grandensis, L. riparia, and L. booriae [1,6]. Among these species, L. monocytogenes and L. ivanovii are considered the most pathogenic species, and L. monocytogenes is responsible for several outbreaks in humans and animals [3,4].
Listeria monocytogenes, first described and isolated by G. Hülphers in 1919, was later identified by E.G.D. Murray in 1923 and J.H. Pirie in 1925. In 1940, it was recognized as L. monocytogenes [1,7,8]. Nyfeldt first isolated it in humans in 1929 and later described the circling diseases caused by it in sheep [1]. Listeriosis is characterized as a zoonotic disease resulting from the ingestion of contaminated food by L. monocytogenes. Systemic dissemination of pathogens from the gastrointestinal tract depends on their ability to overcome barriers such as the intestinal, blood-brain, and placental barriers [1,9]. Listeriosis is characterized by septisis and central nervous system infections, occurring primarily in immunocompromised hosts, the elderly, and pregnant women, as well as localized infections anatomically rare. Gastroenteritis is caused by healthy individuals when the ingested contaminated ready-to-eat foods such as hotdogs, cheeses (unpasteurised milk), smoked fish, ice cream, patés, cantaloupe, apple, and vegetables [9,10]. Although morbidity is very low in the normal population, these epidemics are characterized by high hospitalization and mortality rates, especially in high-risk groups with hospitalization rates higher than 95% in these cases [1,10]. This microorganism is responsible for 1600 illnesses and 260 deaths annually in the United States, has a zero-tolerance policy due to its higher disease severity [11]. Listeria species, more specific L. monocytogenes is a ubiquitous bacterium (Figure 1) known for its adaptability, including antibiotic resistance genes and biofilm formation [2,10]. Its resistance to adverse environmental conditions such as high salt concentration, temperature range low pH and oxygen-limiting conditions, allows it to spread through food and multiply on various surfaces [9].
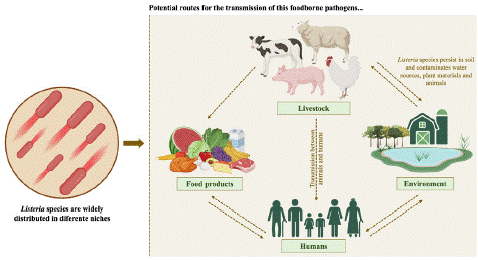
Figure 1: Transmission routes of Listeria species.
Prevalence and Occurrence in Livestock
In this review article, we gathered information resulting mainly from studies that detected Listeria species in livestock animals, such as goats, cattle, buffaloes, sheeps, cows, dairy cattle, chickens, slaughterhouses, poultry farms and pigs are summarized in Table 1.
Location
Listeria Species
Livestock animals
Sample origin
Collected samples
Prevalence (%)
Reference
India
L. monocytogenes
Goat
Faecal
Nasal
Vaginal
95
3.15
6.3
3.1[80]
Latvia
L. monocytogenes
L. innocua
L. seeligeri
L. ivanoviiCattle
Faecal
111
25.2
33.2
15.3
2.7[16]
Egypt
L. monocytogenes
L. ivanovii
L. innocua
L. grayiCattle
Faecal
70
4.3
5.7
2.9
5.7
L. ivanovii
Buffaloes
Faecal
30
6.7
L. innocua
3.3
L. grayi
6.7
L. monocytogenes
Sheep
Faecal
50
8.0
L. ivanovii
6.0
L. innocua
2.0
L. grayi
2.0
L. ivanovii
Goats
Faecal
25
8.0
L. innocua
4.0
Turkey
L. monocytogenes
L. innocua
L. ivanoviiCows
Milk
68
4.41
10.29
4.41[13]
L. monocytogenes
Slaughterhouse A
Chicken
11
0
[55]
Slaughterhouse B
Chicken
12
16.7
Norway
L. monocytogenes
Dairy cattle
Faecal
99
30
[22]
Spain
L. monocytogenes
Dairy Cattle
Faecal
79
65
[81]
Italy
L. monocytogenes
Broiler chicken meat
Neck skin
520
26.7
[82]
Canada
L. monocytogenes
Slaughterhouses A
Slaughterhouses B
Slaughterhouses C
Slaughterhouses DSwine meat
624
9.3
8.8
15.7
5.1[83]
USA
L. monocytogenes
Dairy cattle
PoultryManure
67
13.1
2.2[56]
L. monocytogenes
Poultry farms
Faecal
1537
1.8
[84]
Nigeria
L.monocytogenes
L.ivanovii
L.grayi
L. innocuaBroiler chickens
Faecal
114
13.2
7.9
2.6
2.6[65]
Germany
L.monocytogenes
L. innocuaSlaughter pigs
Tonsil
430
1.6
1.2[19]
Table 1: Prevalence of Listeria species reported livestock animals.
Listeria species are widely disseminated in the environmental, but infection in farm animals can occur when grazing on contaminated fields or fields fertilized with contaminated manure [12,13]. Contaminated food and livestock are the source of many foodborne pathogens, and L. monocytogenes has been documented in a broad range of animal species, but commonly affect livestock animals and is responsible for listeriosis in animals and humans [14,15]. L. ivanovii, also is associated with animal infections and are found to be the most pathogenic along with L. monocytogenes [16]. Infections caused by Listeria spp. have a negative impact on the livestock economy as well as the food processing industry, including human health [12].
Regarding the prevalence of Listeria spp. found in the livestock environment, five Listeria species have been identified namely L. monocytogenes, L. innocua, L. seeligeri and L. ivanovii has been isolated from all types of samples such as faecal, nasal, vaginal, milk, skins, meat, manure and tosil. L. monocytogenes showed the highest prevalence and was isolated in most studies in Table 1. According to Castro et al. (2018), dairy cattle farms have been identified as significant reservoirs of L. monocytogenes genotypes associated with human listeriosis outbreaks [14]. However, there are other causes for these contaminations and transmission of L. monocytogenes to the farm environment from a multitude of sources, like poor-quality silage [17].
The prevalence of Listeria spp. infection in bovine/cattle farm environments is often high, including subtypes associated with human infections and foodborne outbreaks, mainly detected in feces and feeding units [18]. In Table 1, is possible to observe six studies related on bovine/cattle farming in various regions of the world such as Latvia, Egypt, Turkey, Norway, Spain and US, showing rates of Listeria spp. infection ranging from 65% to 2%. The presence of different species has also been confirmed., with L. innocua being the most common in Turkey and Latvia, followed by L. monocytogenes in Norway and Spain. The prevalence rate in the USA was 13% and the prevalence of Listeria spp. in cattle environments confirms that this is an important reservoir of the species. Livestock likely spread pathogens asymptomatically, showing no signs of illness or shedding bacteria into the farm environmen [16].
In pig production, the main problem caused by this pathogenic bacteria is the fact that animals can carry the bacteria without showing any signs of disease at slaughter, leading to direct contamination of carcasses and meat at the slaughterhouse [14,18]. The study in Germany found low prevalence of Listeria species, with 1.6% for L. monocytogenes and 1.6% for L. innocua. However, it suggests that tonsil samples can harbor these species, potentially posing a risk of cross-contamination and food chain spread [19].
In avian farms (chickens, turkeys, waterfowl, geese, ducks, game birds, pigeons, parrots, etc.), the Listeria spp. outbreaks are rare and are more frequently reported as an opportunistic pathogen, however, are important potential vectors for contamination of the processing environment. Sporadic cases of listeriosis have been attributed to poultry, indicating that poultry can serve as a potential source of Listeria spp. infection in humans. Listeria spp. have been isolated from various stages of the poultry production and processing continuum [14,20]. Although, as described, this bacterium is not common in poultry, we can see six studies in our Table 1 in which Listeria spp. was isolated from different sources, such as manure, faecal and meat samples. The most common species is L. monocytogenes with a prevalence ranging from 27% and 0%. Other three species were also detected in the study carried out in Nigeria [21], namely L.ivanovii, L.grayi and L. innocua, with low prevalence.
Improper hygienic practices are strongly associated with the presence of this species in livestock, suggesting that good hygiene is not the only important factor in livestock and that the majority contamination comes from animal and environmental sources. Therefore, all the implementing measures established in farms and their surroundings at every stage of production are of critical importance to have a significant impact in food safety in the future [14,16,22].
Antimicrobial Resistance in L. monocytogenes: Emerging Crisis
Antimicrobial resistance is an emerging threat to public health and the development of antibiotic resistance in bacteria has been associated to the use and misuse of antimicrobial in human health and veterinary [23,24]. The use of antibiotics in food animals is common and has been used on a large scale for long periods of time. As a result, a positive selection of resistant bacterial clones can spread to humans through the food chain, with bacteria acquiring a wide variety of antibiotic resistance genes. Commensal organisms found in food or in the gastrointestinal tract of animals and humans that can be a possible way of contamination for Listeria spp. and other pathogens [23,25].
Therapeutic options including the use of β-lactam (penicillin or ampicillin) or in combination with an aminoglycoside, mainly gentamicin and amoxicillin [26,27], are the antibiotics selected in the treatment of severe infections or if the patient is allergic to β-lactams. In the case of resistance to the antibiotics like fluoroquinolones, macrolides and tetracyclines, trimethoprim-sulfamethoxazole are successfully used to treat listeriosis [10,26]. The use of antibiotics in human medicine, veterinary medicine, and agriculture actually plays an important role in the emergence and spread of antibiotic resistance [28]. In livestock, veterinarians, and farmers play an important role in the use of antibiotics. Antibiotics are often used both therapeutically and sub therapeutically for the treatment and prevention (prophylaxis) of bacterial diseases in animals [29–31]. The use antimicrobials at industrial scale for growth promotion purposes in animal agriculture has been a significant concern. Since 2006, European Union (EU) countries and World Organisation for Animal Health (WOAH) banned the use of antimicrobial as growth promoters in animal feed and is believed that the use of antimicrobials is one of the major contributor to the global trend of antimicrobial resistance since they have been used for at least 50 years [28,30,32].
In fact, β-lactams, tetracyclines, aminoglycosides, lincosamides, quinolones, polypeptides, amphenicols, macrolides, and sulfonamides are indeed among the most commonly used classes of antibiotics in food animal production [33]. For cattle, poultry, and pigs, the estimated average annual consumption of antimicrobials is 45 mg/kg, 148 mg/kg, and 172 mg/kg, respectively. Global antibiotic consumption is projected to increase, estimated to increase from 63,151 ± 1,560 tons to 105,596 ± 3,605 tons by 2030 [32]. Predictable patterns of intensification in food systems often correlate with increased demand for antimicrobial use [31,33]. As a result, bacteria present in food animals often proliferate in fresh meat and milk and dairy products and can act as a reservoir for resistance genes that can be transferred to humans [32,33]. The first case of antibiotic resistance in a food animal was reported after streptomycin was administered to turkeys in 1951. As a result, widespread resistance to antibiotics such as tetracyclines, sulfonamides, β-lactams, and penicillin, has been observed in a variety of other food-producing animals [32].
Classical microbiological methods and molecular techniques are of great importance for testing Listeria monocytogenes in food and manufacturing environments. Molecular-based approaches offer improved discriminatory power for differentiating bacterial strains in epidemiological studies [34]. Whole Genome Sequencing (WGS) technologies are rapidly developing novel typing methods due to their rapid, sensitivity, and high accuracy. They provide extensive additional information, and exhibiting the highest discriminatory power when comparing various organisms, making them effective in detecting foodborne outbreaks and studying pathogenic bacteria molecular epidemiology, including L. monocytogenes [35-38]. Analyzing bacterial genome sequences provides detailed information about isolates relationships, molecular types and virulence and resistance markers [36,37,39]. This technology is suitable for national and international surveillance systems, enhancing food safety and public health efforts by understanding infectious diseases epidemiology in the future [39]. The study of lineages and clonal complex is crucial for understanding the relationship between genetic variation within a species and traits like pathogenic potential, virulence, and epidemiology [40]. L. monocytogenes exhibits a structured population consisting of 14 serotypes and 4 distinct lineages (I, II III, and IV), which, from an evolutionary perspective, could be regarded as distinct species [41]. Each lineage is characterized by specific serotypes: lineage I includes serotypes 1/2b, 3b, 4b, 4e and 7; lineage II includes serotypes 1/2a, 1/2 c, 3a and 3c; lineage III includes serotypes 4b, 1/2a, 4a and 4c; and lineage IV includes serotypes 4a and 4 c [37,42]. The most predominant serotypes causing clinical infections are 1/2a (lineage II), 1/2b and 4b (lineage I), accounting for over 90% of cases [37]. Notably, serotype 4, belonging to lineage I, is frequently isolated from human infections, indicating its high prevalence and pathogenicity. Serotype 4b is also responsible for a majority of sporadic and outbreak incidents worldwide, further underscoring its elevated pathogenic potential. Strains are organized into Clonal Complexes (CCs) and singletons are Sequence Types (STs) with at least two allelic mismatches [43]. Multilocus Sequence Typing (MLST) can reconstruct ancestral and evolutionary relationships among L. monocytogenes isolates and identify all genetic variations within amplified housekeeping genes, which accumulate over time are less common in human disease, they are frequently found in food and food environments[44]. The lineages are further classified into STs and CCs using Multilocus Sequence Typing (MLST). Lineage I includes the clones CC1, CC2, CC4, and CC6, which are commonly associated with human diseases and dairy products [45], whereas lineage II comprises the clones CC9 and CC121, which are strongly linked to food and food processing environments [41,46].
The monitoring of antimicrobial resistance in zoonotic and commensal bacteria in food-producing animals and food is crucial for several reasons. It helps in understanding the development and spread of antimicrobial resistance. By monitoring resistance patterns, we can identify emerging trends and monitoring provides relevant risk assessment data, and evaluating targeted interventions [23,47]. The first antibiotic-multi-resistant strain (chloramphenicol, erythromycin, streptomycin, and tetracycline) of L. monocytogenes was described in France in 1988. Since then, numerous resistant strains have been identified and isolated from both food and human samples. In 1996, antibiotic resistance of Listeria spp. was isolated from food products such as cheese and pork [25,48,49]. In response to growing antibiotic resistance in foodborne pathogens, the European Union introduced legislation banning the use of antibiotics as animal feed additives in January 2006 [25]. Multidrug resistance is not a common pattern among L. monocytogenes, however, as described is characterized by the ability to develop resistance to antimicrobial agents commonly used in human and animal health. Antimicrobial resistance can occur through various mechanisms, such as target gene mutations (e.g. genes encoding efflux pumps) and the acquisition of genetic elements [49].
There are two main routes for the transmission of antimicrobial resistance between food-producing animals and humans. The first is direct acquisition by contact, which occurs through interaction with food-producing animals or human carriers. A second involves indirect acquisition through the food chain or exposure to an environment with high levels of antimicrobial resistance contamination, such as hospitals, nosocomial acquisition, manure, waste water and agriculture lands [28,50].
Genomic Analysis of L.Monocytogenes in Livestock and Derived Food-Products
Table 2 summarizes various studies that investigated the presence of L. monocytogenes in livestock and food derived for detection of antimicrobial resistance and provides an overview of antimicrobial resistance and genomic analysis. A study conducted by Obaidat e Stringer [51] in Jordan analyzed 32 L. monocytogenes strains isolated from cattle, sheep and goats and observed a high levels of resistance (95%) to ampicillin, penicillin, clindamycin, erythromycin, quinupristin–dalfopristin (88%) and streptomycin (80)%, with a multidrug-resistant profile. Additionaly, more than 70% of the isolates showed resistance to teicoplanin, linezolid, vancomycin, kanamycin, and tetracycline [51]. conducted a study in Morocco [53] and collected and analyzed 520 samples of raw beef bovine and poultry meat. Two L. monocytogenes strains were isolated from each sample of raw bovive meat and raw poultry meat. These strains showed high levels of resistance to amoxicillin/clavulanic acid and erythromycin. This study also revealed the presence of virulence factors in L. monocytogenes strains recovered from the collected samples [52]. In another study conducted in Morocco [53], 140 raw beef samples were analyzed, and 10 L. monocytogenes strains were isolated. The strains were highly resistant to amoxicillin and ampicillin, while moderately resistant ro other antibiotics such as streptomycin, penicillin, erythromycin, vancomycin, tetracycline, and sulfamethoxazole/trimethoprim. However, they were highly susceptible to imipenem, amikacin, gentamicin, kanamycin, sulfamethoxazole, and chloramphenicol. Two separate studies on meat and dairy products were conducted in Spain and Australia. Escolar et al. [23] conducted a study in Spain and identified 7 L. monocytogenes strains. They showed a general tendency to be resistant or intermediate susceptible to eight of the nine antibiotics tested. The most commonly observed resistance was to clindamycin, with lower levels of resistance were observed to tetracycline, ciprofloxacin, ampicillin, penicillin, and gentamicin [23]. A study conducted in Brazil [54], L. monocytogenes in pig slaughterhouse, with resistance most commonly observed to clindamycin, tetracycline, ampicillin and trimethoprim-sulfamethoxazole. The strains exhibiting resistance to all nine antibiotics tested, and showed the highest levels of resistance to these antibiotics, were primarily found in the environment of slaughtered pigs [54]. Limited information is available about antibiotic resistance of L. monocytogenes strains isolated from poultry samples. However, a study conducted in Turkey [55] demonstrated low levels of antibiotic resistance among the strains and resistance to sulfamethoxazole/trimethoprim, penicillin G, and erythromycin observed in 5%, 3%, and 2%, respectively [55]. In the USA [56], a study of antimicrobial resistance in dairy cattle and poultry manure, found that 100% of L. monocytogenes isolates were resistant to at least one of the tested antimicrobial classes tested. As shown in Table 2, the observed resistance was significantly higher. Among the resistance genes, the prevalence of penA (50%), ampC (66.6%), and ermB (28%) genes was higher than the prevalence reported in other studies [56]. The reviewed studies revealed several patterns and trends related to antibiotic resistance and pathogenic properties of L. monocytogenes. Other studies were conducted in different regions of the world, including China [57,58], Poland [59], Central Romania [60], Germany [61,62], and Northern Italy [63]. Evaluation of resistance to various antibiotics was confirmed and the presence of several resistance and virulence genes was confirmed. Analysis of different clonal lineages of the isolates revealed that lineage II was the most common. Regarding STs, several STs corresponding to different clonal complexes were identified. The increase in antibiotic resistance in L. monocytogenes highlights the need to monitor the food chain of all food-producing animals and livestock. Widespread use and overuses of antibiotics at various stages of food production can facilitate the spread of resistant bacteria and multi-drug resistant bacteria that are normally present in the animal production environments and processing chains [64,65].
Location
Animals
Number of isolates
Antimicrobial resistance
Genomic analysis
Reference
Method
Antibiotics
Resistance, %
Antimicrobial genes detected
Method
MLST
Lineage (%)
ST
CC
Jordan
Cattle
Sheep
Goat32
Disk diffusion
Ampicillin
Clindamycin
Penicillin
Erythromycin
Quinupristin/dalfopristin
Streptomycin
Teicoplanin
Linezolid
Vancomycin
Kanamycin
Tetracycline
Gentamicin
Chloramphenicol
Ceftriaxone
Ciprofloxacin96.9
96.9
93.8
93.8
87.5
75.8
75.0
75.0
71.9
71.9
71.9
50.0
43.8
34.4
15.6-
-
-
-
-
[51]
Morocco
Raw bovine meat
1
Disk diffusion
Amoxicillin/clavulanic acid
Ampicillin-
-
-
-
-
-
[52]
Raw poultry meat
1
Amoxicillin/clavulanic
Erythromycin
sulphamethoxazole
Tetracycline-
-
Raw beef meat
10
Ampicillin
Penicillin
Amoxicillin
Sulfamethoxazole
Sulfamethoxazole/ trimethoprim
Gentamicin
Streptomycin
Kanamycin
Tetracycline
Chloramphenicol
Erythromycin
Vancomycin80
50
100
20
3010
50
10
50
10
50
50-
[53]
Spain
Meat and dairy products
7
Microdilution
Clindamycin
Tetracycline
Ciprofloxacin
Ampicillin
Penicillin
Gentamycin90
30
26
16
10
2tet M
PCR
-
-
-
[23]
Australia
Dairy products and
Meat products100
Disk diffusion and microdilution
Ciprofloxacin
Erythromycin2.1
1fosX,lmrB,ermB,fepR
PCR
Lineage II, (56%) Lineage I(43%), Lineage III (1%)
-
CC1
[85]
Brazil
Lairage, slaughtering and cutting room
16
Disk diffusion
Kanamycin
Clindamycin
Tetracycline
Erythromycin
Ampicillin
Penicillin
Sulfamethoxazole- trimethoprim6.2
31.2
6.2
6.2
100
18.6.2
75-
PCR
-
-
-
[64]
South Africa
Raw milk
2
Disk diffusion
Sulfamethoxazole
Trimethoprim
Erythromycin
Cefotetan
Oxytetracycline71.43
52.86
42.86
42.86
42.86blaTEM, blaSHV,blaZ, tetA, tetD, tetG, tetM, tetK, aph(3)-IIa (aphA2)a, sul1, sul2
PCR
-
-
-
[86]
milk/fresh milk
7
Cheese
12
Ready-to-eat products
6
-
-
-
EmrB/QacA, Bcr/CflA, SugE, Tn6188, bcrABC, fosX, tetA, tetM, mecC, mrB, msrA, lde, mdrL.
Databases
lineage II and lineage
ST1, ST121, ST204,
ST876-
[68]
Turkey
Poultry Slaughterhouse A
11
Disk diffusion
Sulfamethoxazole/trimethoprim
Penicillin G
Erythromycin5
3
2-
PCR
Lineage I (90.26%, Lineage II (5.82%), Lineage III (3.88%)
-
-
[55]
Poultry Slaughterhouse B
12
Disk diffusion
USA
Dairy Manure
58
Broth microdilution method
Ampicillin
Penicillin G
Chloramphenicol
Ciprofloxacin
Nalidixic acid
Kanamycin
Gentamycin
Streptomycin
Tetracycline
Erythromycin
Azithromycin
Ceftriaxone
Cefoxitin
Trimethoprim/ Sulfamethoxazole
Meropenem
Vancomycin
Linezolid
Nitrofurantoin
Clindamycin
Rifampicin
Levofloxacin89.5
47.7
61.7
79
95.5
88
77.6
98.5
34.3
37.3
100
100
100
100100
67
58
8.9
100
100
91Lde, ampC, aadB, penA, ermB, tet(O)
PCR
-
-
-
[56]
Poultry Manure
9
Broth microdilution method
Manure
47
Soil
20
China
Food (frozen beef, frozen pork, fresh fish, fresh aquatic products, frozen chicken, frozen sheep casing and dairy food products)
101
Microdilution method
Oxacillin
Clindamycin
Daptomycin
Chloramphenicol
Tetracycline
Ciprofloxacin
Erythromycin
imipenem39.33
16.85
6.74
5.62
4.49
3.37
3.37
1.12aph(4)Ia, ermC, fexA, tetK, tetM, tetM, tetK; fexA, ermC
PCR
lineage II (64.20 %); lineage I (35.80 %)
ST9, ST121, ST37, ST8, ST1, ST204, ST31, ST59, ST2, ST3, ST4, ST7, ST155, ST21, ST388, ST5, ST6, ST77, ST191, ST2117, ST224, ST26, ST325, ST426 ST451
-
[57]
Meat products
90
-
-
-
tet(L), tet(M), aph(3')-III, aac(6')-Iaa, str, erm(B), Isa(A), optrA, Cat, fexB, dfrG, sul1, norB, bcrA, aadA3, qnrA2, vanRG
PCR
lineage II andlineage I
ST2, ST9, ST155, ST8, ST121, ST120, ST87, ST196, ST11, ST387, ST705
CC9, CC121, CC155, CC8, CC87, CC2
[58]
Poland
Ready-to-eat products (heat-treated sausages, delicatessen, salads, and packed dinner dishes, Fish seafood.
146
Microbroth dilution method
Oxacillin
Clindamycin
Ceftriaxone
Linezolid
Ciprofloxacin
Gatifloxacin
Gentamycin
tetracycline90.4
54.1
49.3
3.4
0.7
0.7
0.7-
PCR
-
ST9, ST3, ST580, ST1266, ST1267 ST1268)
CC8, CC5, CC9, CC2, CC5, CC8, CC9
[59]
Central Romania
Ready-to-eat processed meet
17
Vitek2 Compact automated system
Benzylpenicillin
fusidic acid
oxacillin
Fosfomycin
Clindamycin
Imipenem
Ciprofloxacin
Rifampin
trimethoprim-sulfamethoxazole
tetracycline100
100
88.2
82.4
76.5
52.9
41.2
41.2
29.4
29.4-
PCR
lineage II (58.9%), lineage I (29.4%), lineage III /11.8)
-
-
[60]
Germany
primary production, processing companies, fresh fruit, frozen berries from supermarkets
8
Broth microdilution
Fosfomycin, cationic peptide, lincomycin, fluoroquinolones
-
fosX, mprF, lin, nor
PCR
lineages I and II (62.5%)
ST1, ST2, ST6, ST7, ST21, ST504, ST1413
CC1, CC2, CC6, CC7, CC21, CC457, CC739
[61]
Fattening pigs and the slaughterhouse environmen
7
Broth microdilution
Clindamycin
Pirlimycin100
100vga(G), fosX
BakCharak pipeline
lineage I and lineage II
ST5, ST6, ST7, ST9, ST18, ST20, ST37, ST325, ST412, ST451
CC5, CC6, CC7, CC9, CC18, CC20, CC37, CC31, CC412, CC11
[62]
Northern Italy
Food sources (beef, dairy, fish, game, mixed food, mixed meat, pork, and poultry)
416
-
-
-
-
-
-
ST1ST2, ST3, ST5, ST9, ST36, ST427, ST663
CC1, CC2, CC3, CC5, CC9, CC36, CC29
[63]
Portugal
Cured Raw Milk Cheese
8
-
-
-
-
-
-
ST788, ST378, ST1, ST9, ST666, ST87
-
[66]
Table 2: Antimicrobial resistance and genomic analysis of L. monocytogenes in different regions.
Multiple studies have been conducted on L. monocytogenes strains, employing the WGS as the primary technique. A study conducted in Portugal by Joana Praça et. al [66], in the analysis of ninety-six cured raw milk cheeses from various batches in the Alentejo region of Portugal, the most frequent clonal complexes observed in L. monocytogenes typing were ST1, ST9, and ST87, which were detected in five isolates. Interestingly, these three complexes have previously been reported by Alexandra Moura et al. [67] and Anaïs Painset et al. [37] in studies involving clinically confirmed L. monocytogenes isolates, as well as in investigations focused on ready-to-eat foods, food-processing environments, and food samples. Moreover, in a study conducted in Spain [68] that identified clinical isolates associated with listeriosis, clonal complexes ST1 and ST87 were also identified as the most prevalent complexes. One study conducted in South Africa [69] isolated six L. monocytogenes strains from a ready-to-eat meat product, especially biltong and polony. Four distinct sequence types were identified: ST1, ST121, ST204, and ST876. It was observed that ST1, which was found in 50% of the isolates, has been reported to have a global distribution [69]. Regarding the ST found, ST1 is known to be commonly found in clinical and food isolates across different regions worldwide [70]. ST121 and ST204, on the other hand, are associated with species typically found in food-processing environments. The particular sequence types possess the capacity to endure and persist for extended durations, ranging from months to years, within food-processing environments, thereby continuing to contaminate food products [71,72]. During a study carried out in South Africa [73], L. monocytogenes was isolated from various stages of the meat value chain, including different types of meat, meat products, and environmental samples. WGS was employed for characterization purposes and the MLST analysis of the isolated strains revealed the presence of 20 distinct sequence types, primarily belonging to lineages I and II. Among the most prevalent STs identified were ST204, ST321, ST1, ST2 and ST9. It is worth noting that ST204 has been previously associated with strains causing food contamination in meat-related products in studies conducted in France [74] and Australia [75]. However, ST204 has been reported and isolates from various ecological niches, including food processing facilities, non-clinical isolates, and ready-to-eat products. On the other hand, ST1 and ST2 are recognized as the predominant sequence types strongly associated with food contamination and responsible for infections in both humans and animals on a global scale [73]. Some STs (ST2, ST3, ST5, ST9, ST155 and ST204) were found to exhibit mechanisms enabling their survival in animal production environments while also contributing to the persistence of food contamination [73,76]. A study conducted in Latvia [16] examined the genetic diversity of L. monocytogenes in cattle farms by analyzing 521 samples collected from 27 cattle farms between 2019 and 2020. Molecular serotyping, Clonal Complexes (CCs), and genetic diversity of the L. monocytogenes isolates were investigated. The results revealed that the majority of the sequenced L. monocytogenes isolates belonged to serogroup IIa, followed by IVb and IIc. Serogroup IIa was detected in various sources, including soil, feed, water, and animal feces, while IVb was found in water and feces, and IIc was only present in feces. Fifteen ST and corresponding CC were identified among the L. monocytogenes isolates. The most abundant STs and CCs were ST37 (CC37), ST451 (CC11), and ST18 (CC37). In the cattle farms, the predominant STs and CCs were ST18 (CC18), ST37 (CC37), and ST8 (CC8). ST37 was significantly associated with soil and was exclusively observed among soil isolates from for different farms and was linked to ruminants, ruminant farms, and wildlife environments [16,77]. Clonal complexes, CC8, CC11 and CC9, were associated with food and persistence in food-processing environments and had been implicated in listeriosis outbreaks [78,79]. CC37 and CC18 clones suggested adaptation and persistence in the cattle farm environment [16].
The mentioned studies provide valuable insights into the genetic diversity, prevalence, and distribution of L. monocytogenes isolates in various settings and geographic regions. The study by Joana Praça et. al [66] in Portugal observed common clonal complexes ST1, ST9, and ST87 in L. monocytogenes isolates from raw milk cheeses, which were previously reported in clinical and food related studies. Similarly, in Spain, the same clonal complexes (ST1 and ST87) were found to be prevalent among clinical isolates associated with listeriosis. In South Africa, multiple studies reported the presence of distinct sequence types (ST1, ST121, ST204, ST876) in L. monocytogenes strains isolated from ready-to-eat meat products and different stages of the meat value chain.
ST1 was recognized as globally distributed and commonly found in clinical and food isolates. ST121 and ST204 were associated with species prevalent in food-processing environments, capable of persisting and contaminating food products. Furthermore, the study conducted in Latvia, ST37, ST451, and ST18 were frequently identified and the ST8, ST11, ST9, and ST37 were associated with food, persistence in food-processing environments, and ruminant farms. The findings collectively indicate the presence of specific ST and CC that exhibit adaptability, persistence, and potential for food contamination in different ecological niches and highlight the significance of L. monocytogenes in various contexts, including food products, clinical infections, food-processing environments, and animal production settings.
Conclusions
The extensive use of antibiotics in human medicine, veterinary medicine, and agriculture has contributed significantly to the emergence and spread of antibiotics resistance. The use of antimicrobials in livestock, particularly for growth promotion purposes, has been a major concern. The presence of antibiotic resistance in food-producing animals, such as cattle, poultry, swines and rabbits, has been well-documented and antibiotics from various classes, including β-lactams, tetracyclines, aminoglycosides, quinolones, and macrolides, are commonly used in food animal production. These antibiotics can lead to the dissemination of antibiotic-resistant bacteria in fresh meat, milk, and dairy products, potentially acting as reservoirs for resistant genes that can be transferred to humans. Studies on L. monocytogenes have shown a high prevalence of multidrug resistance strains and exhibit resistance to antibiotics such as ampicillin, penicillin, clindamycin, erythromycin, and tetracycline. To mitigate the spread of antibiotic resistance and ensure food safety, there is a need for surveillance and monitoring of antibiotic use in food-producing animals and the food chain. Responsible antibiotic stewardship practices, strict adherence to regulations, and promoting alternatives to antibiotics in animal agriculture are crucial steps in combating antibiotic resistance. Continued research, including genomic studies using WGS, will play a significant role in understanding and addressing the challengesposed by antibiotic resistance in foodborne pathogens like L. monocytogenes.
Author Statements
Conflict of Interest
The authors state no conflict of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
This work was supported by the Associate Laboratory for Green Chemistry—LAQV which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020). Adriana Silva is grateful to FCT (Fundac ao para a Ciencia e a Tecnologia) for financial support through the PhD grant SFRH/BD/04576/2020. Funding: This work was supported by the projects UIDP/00772/2020 (Doi: 10.54499/UIDB/00772/2020) funded by the Portuguese Foundation for Science and Technology (FCT).
Author Contributions
Conceptualization, A.S.; validation, P.P., M.T.R., P.V. and V.F.; investigation, V.S. and A.S.; data curation, V.S., J.E.P., L.C. and L.M.; writing—original draft preparation, A.S., G.I. and V.S.; writing—review and editing, A.S.; All authors have read and agreed to the published version of the manuscript.
References
- Matle I, Mbatha KR, Madoroba E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort Journal of Veterinary Research. 2020; 87: a1869.
- Abatcha MG, Goni MD, Abbas MA. A Review of Listeria and Salmonella: An Update on Description, Characteristics, Incidence, and Antibiotic Susceptibility. Adv Anim Vet Sci. 2020.
- Kaptchouang Tchatchouand CD, Fri J, Montso PK. Evidence of Virulent Multi-Drug Resistant and Biofilm-Forming Listeria Species Isolated from Various Sources in South Africa. Pathogens. 2022; 11: 843.
- Mpondo L, Ebomah KE, Okoh AI. Multidrug-Resistant Listeria Species Shows Abundance in Environmental Waters of a Key District Municipality in South Africa. IJERPH. 2021; 18: 481.
- Quereda JJ, Morón-García A, Palacios-Gorba C. Pathogenicity and virulence of Listeria monocytogenes : A trip from environmental to medical microbiology. Virulence. 2021; 12: 2509–45.
- Gebremedhin EZ, Hirpa G, Borana BM. Listeria Species Occurrence and Associated Factors and Antibiogram of Listeria monocytogenes in Beef at Abattoirs, Butchers, and Restaurants in Ambo and Holeta in Ethiopia. IDR. 2021; 14: 1493–504.
- Saodat H. Immune Reactions in the Body Based on Listeriosis. 2022; 7.
- Disson O, Moura A, Lecuit M. Molecular characteristics and virulence potential of Listeria monocytogenes isolates from Chinese food systems. Trends in Microbiology. 2021; 29: 811–22.
- Lopes-Luz L, Mendonça M, Bernardes Fogaça M. Listeria monocytogenes: review of pathogenesis and virulence determinants-targeted immunological assays. Critical Reviews in Microbiology. 2021; 47: 647–66.
- Mackiw E, Stasiak M, Kowalska J. Occurrence and Characteristics of Listeria monocytogenes in Ready-to-Eat Meat Products in Poland. Journal of Food Protection. 2020; 83: 1002–9.
- Townsend A, Strawn LK, Chapman BJ, Dunn LL. A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain. Foods. 2021; 10: 1427.
- Farag H, Abdallah M, Nossair M. Prevalence of Listeriosis in some farm animals. Damanhour Journal of Veterinary Sciences. 2021; 6: 17–20.
- Babacan O. Determination of the presence and antibiotic resistance of Listeria species and aerobic mesophilic bacteria count of cow milks. Veteriner Hekimler Dernegi Dergisi. 2020.
- Rodriguez C, Taminiau B, García-Fuentes E. Listeria monocytogenes dissemination in farming and primary production: Sources, shedding and control measures. Food Control. 2021; 120: 107540.
- Anwar TM, Pan H, Chai W. Genetic diversity, virulence factors, and antimicrobial resistance of Listeria monocytogenes from food, livestock, and clinical samples between 2002 and 2019 in China. International Journal of Food Microbiology. 2022; 366: 109572.
- Terentjeva M, Šteingolde Ž, Meistere I. Prevalence, Genetic Diversity and Factors Associated with Distribution of Listeria monocytogenes and Other Listeria spp. in Cattle Farms in Latvia. Pathogens. 2021; 10: 851.
- Castro H, Jaakkonen A, Hakkinen M. Occurrence, Persistence, and Contamination Routes of Listeria monocytogenes Genotypes on Three Finnish Dairy Cattle Farms: a Longitudinal Study. Appl Environ Microbiol. 2018; 84: e02000-17.
- Stein H, Stessl B, Brunthaler R. Listeriosis in fattening pigs caused by poor quality silage - a case report. BMC Vet Res. 2018; 14: 362.
- Oswaldi V, Dzierzon J, Thieme S. Slaughter pigs as carrier of Listeria monocytogenes in Germany. J Consum Prot Food Saf. 2021; 16: 109-115.
- Rothrock MJ, Davis ML, Locatelli A. Listeria Occurrence in Poultry Flocks: Detection and Potential Implications. Front Vet Sci. 2017; 4.
- Chibuike KU, Iroha IR, Moses IB. Phenotypic screening of multidrug-resistant E. coli from water and fish collected from different fish farms within Abakaliki metropolis, Nigeria. Sci Res Essays. 2021; 16: 15–9.
- Idland L, Granquist EG, Aspholm M, Lindbäck T. The prevalence of Campylobacter spp., Listeria monocytogenes and Shiga toxin-producing Escherichia coli in Norwegian dairy cattle farms: A comparison between free stall and tie stall housing systems. Journal of Applied Microbiology. 2022; 132: 3959–72.
- Escolar C, Gómez D, Carmen Rota García M. Antimicrobial Resistance Profiles of Listeria monocytogenes and Listeria innocua Isolated from Ready-to-Eat Products of Animal Origin in Spain. Foodborne Pathogens and Disease. 2017; 14: 357–63.
- Charpentier E, Courvalin P. Antibiotic Resistance in Listeria spp. Antimicrob Agents Chemother. 1999; 43: 2103–8.
- Lungu B, O’Bryan CA, Muthaiyan A. Listeria monocytogenes : Antibiotic Resistance in Food Production. Foodborne Pathogens and Disease. 2011; 8: 569–78.
- Khademi F, Sahebkar A. A systematic review and meta-analysis on the prevalence of antibiotic-resistant Listeria species in food, animal and human specimens in Iran. J Food Sci Technol. 2019; 56: 5167–83.
- Walsh D, Duffy G, Sheridan JJ. Antibiotic resistance among Listeria, including Listeria monocytogenes, in retail foods. J Appl Microbiol. 2001; 90: 517–22.
- Vidovic N, Vidovic S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics. 2020; 9: 52.
- Magouras I, Carmo LP, Stärk KDC, Schüpbach-Regula G. Antimicrobial Usage and -Resistance in Livestock: Where Should We Focus?. Front Vet Sci. 2017; 4: 148.
- Wee BA, Muloi DM, Bunnik BAD. Quantifying the transmission of antimicrobial resistance at the human and livestock interface with genomics. Clinical Microbiology and Infection. 2020; 26: 1612–6.
- Gilbert W, Thomas LF, Coyne L, Rushton J. Review: Mitigating the risks posed by intensification in livestock production: the examples of antimicrobial resistance and zoonoses. animal. 2021; 15: 100123.
- Ma F, Xu S, Tang Z. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health. 2021; 3: 32–8.
- Zahangir Hosain MD, Lutful Kabir SM, Mostofa Kamal MD. Antimicrobial uses for livestock production in developing countries. Vet World. 2021; 14: 210–21.
- Oniciuc E, Likotrafiti E, Alvarez-Molina A. The Present and Future of Whole Genome Sequencing (WGS) and Whole Metagenome Sequencing (WMS) for Surveillance of Antimicrobial Resistant Microorganisms and Antimicrobial Resistance Genes across the Food Chain. Genes. 2018; 9: 268.
- Šteingolde Ž, Meistere I, Avsejenko J. Characterization and Genetic Diversity of Listeria monocytogenes Isolated from Cattle Abortions in Latvia, 2013–2018. Veterinary Sciences. 2021; 8: 195.
- Wieczorek K, Bomba A, Osek J. Whole-Genome Sequencing-Based Characterization of Listeria monocytogenes from Fish and Fish Production Environments in Poland. IJMS. 2020; 21: 9419.
- Painset A, Björkman JT, Kiil K. LiSEQ – whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microbial Genomics. 2019: 5.
- Authority EFS, Fierro RG, Thomas-Lopez D, Deserio D, Liebana E, Rizzi V, et al. Outcome of EC/EFSA questionnaire (2016) on use of Whole Genome Sequencing (WGS) for food- and waterborne pathogens isolated from animals, food, feed and related environmental samples in EU/EFTA countries. EFS3. 2018; 15: 1432E.
- Brown E, Dessai U, McGarry S, Gerner-Smidt P. Use of Whole-Genome Sequencing for Food Safety and Public Health in the United States. Foodborne Pathogens and Disease. 2019; 16: 441–50.
- Cantinelli T, Chenal-Francisque V, Diancourt L. Epidemic Clones” of Listeria monocytogenes Are Widespread and Ancient Clonal Groups. J Clin Microbiol. 2013; 51: 3770–9.
- Fagerlund A, Idland L, Heir E. Whole-Genome Sequencing Analysis of Listeria monocytogenes from Rural, Urban, and Farm Environments in Norway: Genetic Diversity, Persistence, and Relation to Clinical and Food Isolates. Appl Environ Microbiol. 2022; 88: e02136-21.
- Burnett E, Kucerova Z, Freeman M. Whole-Genome Sequencing Reveals Multiple Subpopulations of Dominant and Persistent Lineage I Isolates of Listeria monocytogenes in Two Meat Processing Facilities during 2011–2015. Microorganisms. 2022; 10: 1070.
- Ragon M, Wirth T, Hollandt F, Lavenir R, Lecuit M, Monnier AL, et al. A New Perspective on Listeria monocytogenes Evolution. 2008.
- Stessl B, Rückerl I, Wagner M. Multilocus Sequence Typing (MLST) of Listeria monocytogenes. In: Jordan K, Fox EM, Wagner M, editors. Listeria monocytogenes. New York, New York, NY: Springer. 2014; 1157: 73–83.
- Maury MM, Bracq-Dieye H, Huang L, Vales G, Lavina M, Thouvenot P, et al. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat Commun. 2019; 10: 2488.
- Kurpas M, Osek J, Moura A. Genomic Characterization of Listeria monocytogenes Isolated from Ready-to-Eat Meat and Meat Processing Environments in Poland. Front Microbiol. 2020: 11.
- European Food Safety Authority, European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. 2015; 13: 4329.
- Moghadam A, Larsen H. Importance of Listeria monocytogenes in food safety: a review of its prevalence, detection, and antibiotic resistance. Iranian Journal of Veterinary Research. 2019; 20: 241-254.
- Luque-Sastre L, Arroyo C, Fox EM, McMahon BJ, Bai L, Li F, et al. Antimicrobial Resistance in Listeria Species. Microbiol Spectr. 2018: 6
- Silva V, Araújo S, Monteiro A. Eira J, Pereira JE, Maltez L, et al. Staphylococcus aureus and MRSA in Livestock: Antimicrobial Resistance and Genetic Lineages. Microorganisms. 2023; 11: 124.
- Obaidat MM, Stringer AP. Prevalence, molecular characterization, and antimicrobial resistance profiles of Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157:H7 on dairy cattle farms in Jordan. Journal of Dairy Science. 2019; 102: 8710–20.
- Bouymajane A, Rhazi Filali F, Oulghazi S. Occurrence, antimicrobial resistance, serotyping and virulence genes of Listeria monocytogenes isolated from foods. Heliyon. 2021; 7: e06169.
- Boukili M, Rhazi Filali F, Lafkih N. Prevalence, characterization and antimicrobial resistance of Listeria monocytogenes isolated from beef meat in Meknes city, Morocco. Germs. 2020; 10: 74–80.
- Rugna G, Carra E, Bergamini F. Distribution, virulence, genotypic characteristics and antibiotic resistance of Listeria monocytogenes isolated over one-year monitoring from two pig slaughterhouses and processing plants and their fresh hams. International Journal of Food Microbiology. 2021; 336: 108912.
- Coban A, Pennone V, Sudagidan M. Prevalence, virulence characterization, and genetic relatedness of Listeria monocytogenes isolated from chicken retail points and poultry slaughterhouses in Turkey. Braz J Microbiol. 2019; 50: 1063–73.
- Hailu W, Helmy YA, Carney-Knisely G. Prevalence and Antimicrobial Resistance Profiles of Foodborne Pathogens Isolated from Dairy Cattle and Poultry Manure Amended Farms in Northeastern Ohio, the United States. Antibiotics. 2021; 10: 1450.
- Shen J, Zhang G, Yang J. Prevalence, antibiotic resistance, and molecular epidemiology of Listeria monocytogenes isolated from imported foods in China during 2018 to 2020. International Journal of Food Microbiology. 2022; 382: 109916.
- Zhang Y, Zhang J, Chang X. Analysis of 90 Listeria monocytogenes contaminated in poultry and livestock meat through whole-genome sequencing. Food Research International. 2022; 159: 111641.
- Sosnowski M, Lachtara B, Wieczorek K, Osek J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. International Journal of Food Microbiology. 2019; 289: 1–6.
- TîRziu E, Herman V, Nichita I. Diversity and Antibiotic Resistance Profiles of Listeria monocytogenes Serogroups in Different Food Products from the Transylvania Region of Central Romania. Journal of Food Protection. 2022; 85: 54–9.
- Wartha S, Bretschneider N, Dangel A. Genetic Characterization of Listeria from Food of Non-Animal Origin Products and from Producing and Processing Companies in Bavaria, Germany. Foods [Internet]. 2023; 12: 1120.
- Oswaldi V, Lüth S, Dzierzon J. Distribution and Characteristics of Listeria spp. in Pigs and Pork Production Chains in Germany. Microorganisms. 2022; 10: 512.
- Filipello V, Mughini-Gras L, Gallina S. Attribution of Listeria monocytogenes human infections to food and animal sources in Northern Italy. Food Microbiology. 2020; 89: 103433.
- Sereno MJ, Viana C, Pegoraro K. Distribution, adhesion, virulence and antibiotic resistance of persistent Listeria monocytogenes in a pig slaughterhouse in Brazil. Food Microbiology. 2019; 84: 103234.
- Chukwu EE, Ibeh VN, Davies-Bolorunduro OF. Occurrence of Multi-Drug Resistant Listeria species in Faecal Samples of Poultry Chickens in Rural Farms in Lagos State, Nigeria. AID. 2021; 11: 49–59.
- Praça J, Furtado R, Coelho A. Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms [Internet]. 2023; 11: 322.
- Moura A, Criscuolo A, Pouseele H. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat Microbiol. 2016; 2: 16185.
- Vallejo P, Cilla G, López-Olaizola M. Epidemiology and Clinical Features of Listeriosis in Gipuzkoa, Spain, 2010–2020. Front Microbiol. 2022; 13: 894334.
- Matle P, Pierneef R, Mbatha R, Magwedere K, Madoroba E. Genomic Diversity of Common Sequence Types of Listeria monocytogenes Isolated from Ready-to-Eat Products of Animal Origin in South Africa. Genes. 2019; 10: 1007.
- Schmitz-Esser S, Müller A, Stessl B, Wagner M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front Microbiol. 2015: 6.
- Wang Y, Zhao A, Zhu R. Genetic diversity and molecular typing of Listeria monocytogenes in China. BMC Microbiol. 2012; 12: 119.
- Smith AM, Tau NP, Smouse SL. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates. Foodborne Pathogens and Disease. 2019; 16: 524–30.
- Matle I, Mafuna T, Madoroba E. Population Structure of Non-ST6 Listeria monocytogenes Isolated in the Red Meat and Poultry Value Chain in South Africa. Microorganisms. 2020; 8: 1152.
- Ebner R, Stephan R, Althaus D. Phenotypic and genotypic characteristics of Listeria monocytogenes strains isolated during 2011–2014 from different food matrices in Switzerland. Food Control. 2015; 57: 321–6.
- Kwong JC, Mercoulia K, Tomita T. Prospective Whole-Genome Sequencing Enhances National Surveillance of Listeria monocytogenes. J Clin Microbiol. 2016; 54: 333–42.
- De Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Krik M, et al. The global burden of listeriosis: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2014; 14: 1073–82.
- Kim SW, Haendiges J, Keller EN, Myers R, Kim A, Lombard JE, et al. Genetic diversity and virulence profiles of Listeria monocytogenes recovered from bulk tank milk, milk filters, and milking equipment from dairies in the United States. PLoS One. 2018; 13: e0197053.
- Chen Y, Gonzalez-Escalona N, Hammack TS. Core Genome Multilocus Sequence Typing for Identification of Globally Distributed Clonal Groups and Differentiation of Outbreak Strains of Listeria monocytogenes Appl Environ Microbiol. 2016; 82: 6258–6272.
- Knudsen GM, Nielsen JB, Marvig RL. Genome-wide-analyses of Listeria monocytogenes from food-processing plants reveals clonal diversity and dates the emergence of persisting sequence types. Environmental Microbiology Reports. 2017; 9: 428–40.
- Kulesh R, Shinde SV, Khan WA. The occurrence of Listeria monocytogenes in goats, farm environment and invertebrates. Biological Rhythm Research. 2022; 53: 831–40.
- Varsaki A, Ortiz S, Santorum P, Lopez P, Lopez-Alonso V, Hernandez M, et al. Prevalence and Population Diversity of Listeria monocytogenes Isolated from Dairy Cattle Farms in the Cantabria Region of Spain. Animals. 2022; 12: 2477.
- Iannetti L, Schirone M, Neri D, Visciano P, Acciari VA, Centorotola G, et al. Listeria monocytogenes in poultry: Detection and strain characterization along an integrated production chain in Italy. Food Microbiology. 2020; 91: 103533.
- Cherifi T, Arsenault J, Pagotto F, Quessy S, Cote JC, Neira K, et al. Distribution, diversity and persistence of Listeria monocytogenes in swine slaughterhouses and their association with food and human listeriosis strains. PLoS One. 2020; 15: e023807.
- Golden CE, Rothrock MJ, Mishra A. Comparison between random forest and gradient boosting machine methods for predicting Listeria spp. prevalence in the environment of pastured poultry farms. Food Research International. 2019; 122: 47–55.
- Wilson A, Gray J, Chandry P, Fox E. Phenotypic and Genotypic Analysis of Antimicrobial Resistance among Listeria monocytogenes Isolated from Australian Food Production Chains. Genes. 2018; 9: 80.
- Kayode AJ, Okoh AI. Assessment of multidrug-resistant Listeria monocytogenes in milk and milk product and One Health perspective. 2022.