
Review Article
J Bacteriol Mycol. 2024; 11 (2): 1219.
Epidemiology and Risk Factors of Infectious Bursal Disease: A Review
Abay Beshah¹; Abdi Ahmed²*; Morka Dandecha¹
1Ambo University, Department of Veterinary Laboratory Technology, Ethiopia
2Animal Health Institute (AHI), Microbiology Research Laboratory, Ethiopia
*Corresponding author: Abdi Ahmed Animal Health Institute (AHI), Microbiology Research Laboratory, P.O. Box 04, Sebeta, Ethiopia. Email: abdivet2014@gmail.com
Received: April 02, 2024 Accepted: May 10, 2024 Published: May 17, 2024
Abstract
Infectious Bursal Disease (IBD) has been a great challenge to the poultry industry world-wide for a long time and has a major setback to productivity and profitability in the poultry industries of developing nations including Ethiopia. In Ethiopia IBDV was spread to almost all the regions and agro-ecological zones and a recent country wide study reported IBDV seropositivity rates in backyard chickens to be close to 95.7%. The infectious bursal disease is widespread viral diseases that affect chicken kept in commercial and backyard production system. Infectious bursal disease virus is a primary affect bursa fabricius of young chicks at the age of 3 to 6 weeks. The most common mode of infection is through the oral route, but conjunctiva and respiratory routes may also be involved. The infectious bursal disease is host-specific and extremely contagious. Age, breed, degree of passive immunity, the virulence of the strain of the virus, biosecurity, and secondary infections associated with the immunosuppressive effects are the most affecting risk factors of this disease worldwide. Although, many studies have been done as world and in Ethiopia concerning the prevalence and identifying predisposing factors of this disease, further experiment on developing vaccines of specific strains and implementation of prevention and control program are needed to be emphasized.
Keywords: Chicks; Epidemiology; Infectious bursal disease virus; Risk factors
Abbreviations: BF: Bursa of Fabricius; CSA: Central Statistical Agency; dsRNA: Double Stranded RNA; DXV: Drosophila X Virus; IBD: Infectious Bursal Disease; IBDV: Infectious Bursal Disease Virus; IPNV: Infectious pancreatic Necrosis Virus; MDA: Maternally Derived Antibody; NAHDIC: National Animal Health Diagnosis and Investigation Center; NCD: Newcastle Diseases; Nm: Nano Meter; OIE: Office of International Des Epizooties; PI: Post Inoculation; SPF: Specific-Pathogen-Free; USA: United State of America; Vv: Very Virulent; vv IBD: Very Virulent Infectious Bursal Disease
Introduction
The poultry sector is one of the segments of livestock sector in Ethiopia which can be characterized into three major production systems: large commercial, small scale commercial and village or backyard poultry production system. These production systems have their own specific chicken breeds, inputs and production properties. Each can sustainability coexist and contribute to solve the socioeconomic problems of different target societies [73]. Ethiopia has 60.51 million chickens population from which 94.33% of the chicken populations are indigenous chickens, while the remaining 3.21% and 2.47% consists of exotic and hybrid breeds [11]. They play a role by providing the needed animal protein that contributes to the improvement of the nutritional status of the people [77].
Ethiopian poultry production has a long traditional practice which is characterized by low input and low output [51]. This indigenous poultry production contributes 98.5 and 99.2% of the national egg and poultry meat production, respectively [73]. Chickens are especially important to women, children and aged individuals, who are the most vulnerable member of the society in terms of under nutrition and poverty; contribute a significant role in supplying animal origin protein to improve human nutrition [27]. Despite, Ethiopia owned huge chicken flock; there are different constraints like poor nutrition, poor management and prevalent diseases that hinder the productivity of the chicken in most area of the country [16].
Among the above obstacles, poultry diseases are the main constraints incriminated for reduction of total numbers and compromised productivity [5]. Among those diseases, Infectious bursal disease is the one that become a serious threat to cause frequent outbreaks and a challenge to the young growing poultry farms [72]. Infectious Bursal Disease Virus (IBDV) is the aetiological agent of Infectious Bursal Disease (IBD), also known as infections bursitis or avian nephrosis. It is a highly contagious disease of young chickens, usually between three and six weeks of age, characterized by high morbidity and mortality [17]. IBD is belongs to the Birnaviridae family and has a non-enveloped, bi-segmented, double-stranded RNA genome which contains a single-shelled, icosahedral capsid structured and having a diameter of 58 nm -60 nm. This relatively simple structure renders the virus very resistant to the outside environment [35]. Infectious bursal disease virus replicates in differentiating lymphocytes of the Bursa of Fabricius, causing the immunosuppressive and often fatal condition called Infectious Bursal Disease (IBD) or Gumboro. IBDV consists of two serotypes (Serotype 1 and 2). Only serotype I viruses are naturally pathogenic to chickens whereas serotype 2 virus apathogenic for chicks [33] and are classified as avirulent, classical, variant and very virulent (vv) strains [50].
The disease is characterized with a typical clinical sign of those an acute immunodepression, with depression, prostration of the affected birds, diarrhoea, during the first weeks of life. It is transmitted through orally via contaminated feed and water [69].
Infectious bursal disease is a newly emerging disease of chicken in Ethiopia, which has been speculated to be introduced concurrent with increased number of commercial state and private poultry farms flourishing in the country [3]. The first report of IBD in Ethiopia was in 2005 involving 20–45 day old broiler and layer chickens from commercial farms [85].
IBD is a disease of worldwide importance due to the huge losses as a result of opportunistic infections encountered by poultry farmers. The disease is especially a problem in developing countries due to challenges, including, but not limited to lack of appropriate vaccines that would be effective against evolving strains of the virus [48]. There is the existence of large gaps in information of the Epidemiology and Risk factors on Infectious bursal disease among poultry farmers. Subsequently, IBD has become a priority problem in commercial and backyard poultry production system despite of distribution of disease occurs; regular vaccination practices and improved biosecurity measures. Therefore, the objective of this seminar paper is:
¾To review the Epidemiology and Risk factors of Infectious bursal disease.
Literature Review
Background
Infectious bursal disease is also known as Gumboro disease which is a highly contagious disease of young chicken (Gallus gallus domesticus) caused by Infectious Bursal Disease Virus (IBDV) which belongs to a genus AviBirnavirus [23], of family Birnaviridae [14] that causes disease and mortality in young chickens mainly 3–6-week-old in the worldwide distribution [41]. The disease is characterized by sudden of short course and extensive destruction of lymphocyte particularly in the bursa of fabricius, where B lymphocytes mature and differentiate [66]; however, IBD viral replication also occurs in other lymphoid structures including the spleen, thymus, harderian gland, and cecal tonsils [63]. Initially there was a misconception that the disease was caused by Infectious Bronchitis Virus (IBV); this was because of presence of similar gross changes in the kidneys [39]. However, in subsequent studies, the causative agent for IBD was isolated in embryonated eggs and the disease given the respective name [82].
The causal agent of the disease was first isolated in Gumboro, Delaware in United States of America (USA), and the disease was originally known as Gumboro disease. It is a viral infection, affecting the immune system of poultry, which is the name derived, even if the terms IBD (infectious bursitis) are more accurate descriptions [10]. In the year of 1960 and 1964, the disease observed in most part of the USA and become devastating disease in Europe in the years of 1962 to 1971 [24]. Infectious bursal diseases currently become an international issue, 95 % of the 65 countries that responded to a survey conducted by the [56] that announced the presence of infection [21]. Infectious bursal disease virus has recently been isolated from a sparrow in China suggesting that wild birds could act as carriers [82], including New Zealand which had been free of disease until 1993 [22] and recently the IBD is reported indifferent parts of Ethiopia [3]. The disease has spread to all investigated commercial farms and multiplication centers occurring at an average outbreak rate of 3-4 farms per year. The disease was encountered commonly in backyard poultry production systems as well [47].
Etiology
Infectious Bursal Disease (IBD) is an acute, highly contagious viral infection of young chickens that has lymphoid tissue as its primary target with a special predilection for the bursa of fabricius. It was first recognized as a specific disease entity by Cosgrove in 1962 and was referred to as “avian nephrosis” because of the extreme kidney damage found in birds that succumbed to infection. Since the first outbreaks occurred in the area of Gumboro, Delaware, “Gumboro disease” was a synonym for this disease and is still frequently used [10]. Infectious bursal disease virus, classified in AviBirnavirus genus under the family of viruses called Birnaviridae family, is the causative agent of Infectious bursal disease [47]. The family includes 3 genera: Aquabirnavirus whose type species is Infectious Pancreatic Necrosis Virus (IPNV), which infects fish, mollusks, and crustaceans; AviBirnaviruswhose type species is Infectious Bursal Disease Virus (IBDV), which infects birds; and Entomobirnavirus whose type species is Drosophila X Virus (DXV), which infects insects [14].
Infectious bursal disease virus particles are bisegmented, double stranded RNA (dsRNA) genomes, non-enveloped virions, which are packaged into single shelled with diameter of 60 to 70 nm [19]. It is replicates in differentiating lymphocytes of the Bursa of Fabricius, causing the immunosuppressive and often fatal condition called Infectious Bursal Disease (IBD) or Gumboro [50].
Two serotypes of the virus have been described; these are Serotype 1 IBDV strains, pathogenic to chickens [38], whereas serotype 2 strains are non-pathogenic [8]. Serotype 1 IBDV isolates comprise the variant, classical virulent and vvIBDV strains, which wide differ in their pathogenicity to chickens. Variant IBDVs do not cause mortality, whereas the classical strains cause up to 20% mortality [50]. Chickens, especially young chicks at the age of 3 to 6 weeks, are the selected hosts for the serotype I virus [43]. In the case of vvIBDV infection, the age susceptibility is extended which covers the entire growing period in broilers [31]. In addition, it was reported that chickens infected with IBDV at the age of 14 days suffered from greater bursal atrophy and had higher viral RNA copy numbers than those infected on the day of hatching [36].
Pathogenesis; Incubation Period and Clinical Signs
Pathogenesis is defined as the method used by the virus to cause injury to the host with mortality, disease or immunosuppression as a consequence. Chickens acquire IBDV infection orally or by inhalation. The virus is transferred from the gut to the other tissues by phagocytic cells like macrophages. In macrophages of the gut associated tissues, it could be detected as early as 4 hours after oral inoculation using immunofluorescence [50]. The virus then reaches the bursa of Fabricius via the blood where the most extensive virus replication occurs. By 13 hours Post Inoculation (PI) most follicles are positive for virus and by 16 hours PI a second and pronounced viremia occurs accompanied by secondary replication in other organs resulting in disease and death [79].
The incubation period is very short which range from 2 to 3 days. In acute cases, the chickens become tired, prostrated, dehydrated, suffered from watery diarrhea, and feathers are ruffled [54]. Mortality commences on the third day of infection, reaches a peak by day four, then drops rapidly, and the surviving chickens recover a state of apparent health after five to seven days. Moreover, a primary infection may also be in apparent when the viral strain is of low pathogenicity or if maternal antibodies are present [76].
The clinical signs of IBD vary considerably from one farm, region, country or even continent to another [79]. In acute form, birds are prostrated, debilitated, dehydrated, with water diarrhea and swollen vents stained with faeces. In birds below three weeks, the disease is asymptomatic, but birds have bursal atrophy with fibrotic or cystic follicles and lymphocytopaenia before six weeks and are usually susceptible to other infections that would be contained in immunocopetent birds (Figure 1) [54].
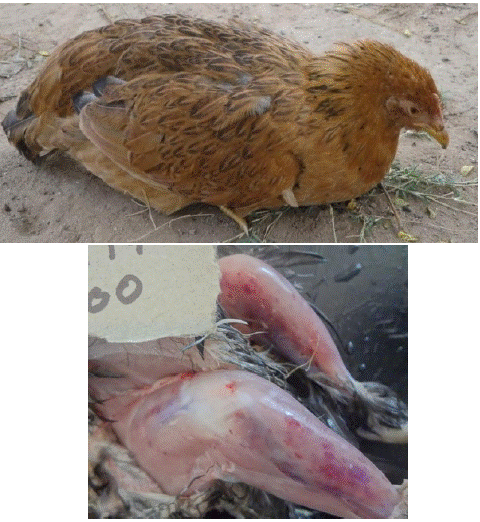
Figure 1: Ruffled feathers in a depressed and Haemorrhages on thigh and leg muscles of an indigenous chicken pullet suffering from infectious bursal disease [54].
Epidemiology
Distribution: Currently, IBDV has a worldwide distribution, occurring in all major poultry producing areas [75]. It was estimated that IBD has considerable socio-economic importance at the international level, as the disease is present in more than 95% of the OIE member countries [20]. Infectious bursal disease is a viral disease regarded as the second most important diseases of village chickens in Africa [1] following Newcastle Disease (NCD). The first outbreak of Infectious Bursal Disease (IBD) that had occurred in 1957 in a broiler farm near Gumboro, the Delaware area in the USA, was caused by the classical serotype 1 IBDV [10]. Infections with serotype 1 IBDV are of worldwide distribution, occurring in all major poultry producing areas. The incidence of infection in the areas where there is serotype 1 is high; essentially, all flocks are exposed to the virus during the early stages of life, either by natural exposure or vaccination [7].
Between 1960 and 1964, the disease affected most regions of the USA and reached Europe in the years 1962 to 1971 [24]. The variant IBDV strains then emerged in the 1980’s in IBDV-vaccinated farms in the Delmarva area and were antigenetically different from the classical strain. Since 1986, Europe has experienced the emergency of vv strain of IBDV, which are characterized by a per acute onset of severe clinical disease and high mortality, which can cause up to 70% flock mortality in laying pullet [78]. Although these new serotype 1 viruses demonstrate increased virulence in their ability to break through the existing level of maternal immunity; they are antigenically similar to the classic strains of IBDV [78]. Strains of vvIBDV have rapidly disseminated to every poultry-producing country such as Middle East, Asia, and Africa, South and Central America in 1999, and in the USA in 2009 were detected [32], but there was no any report that shows the existence of Infectious bursal disease in Canada, Mexico, Australia, and New Zealand [2]. In addition, a survey conducted by World organization for Animal Health (OIE) in 1995, 95% of the 65 countries was responded to declare the case of the infection [21].
In gray, countries where acute forms have been reported. In black, countries where no acute forms have been reported. In white, countries with no report Eterradossi, 1995 as shown in Figure 2.
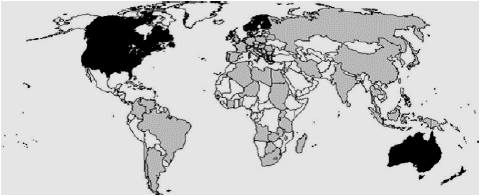
Figure 2: Worldwide geographical distribution of the acute forms of IBDV.
Status of Infectious Bursal Disease in Ethiopia: The disease has spread to all investigated commercial farms and multiplication centers occurring at an average outbreak rate of 3-4 farms per year. The disease was encountered commonly in backyard poultry production systems as well (Ethiopia animal health year book, 2011). According to Ethiopia Animal health year book undertaken during the 2011 fiscal year, Gumboro disease surveillance/investigation was conducted by the NAHDIC in different Regions and they reported that the overall prevalence rates to be about 77.48%. In Ethiopia IBDV was spread to almost all the regions and agro-ecological zones. The first report of IBD in Ethiopia was in 2005 involving 20–45 day old broiler and layer chickens from commercial farms [85]. Since its inception, prevalence of IBD is increasing from year to year and has become a priority problem in backyard poultry production system in Ethiopia, shown in table 1.
Area of study
Sample type
Title
Prev. (%)
Author and Year
Debrezeit
Blood
Investigation on Infectious Bursal Disease Outbreak
49.90%
Aschalew et al., 2005
Debrezeit
Blood
Investigation on infectious bursal disease outbreak
93.30%
Zeleke et al.,2005a
Andassa poultry farm (Amahara region)
Blood
Infectious Bursal Disease case report
100%
Solomon and Abebe, 2007
Andasa poultry farm
Blood
Infectious Bursal Disease case report
72%
Woldemariam and Wossene ,2007
Weast shoa and South west shoa
Blood
Seroprevalence of infectious bursal disease in backyard chickens.
76.64%
Hailu et al., 2010
Mekele
Blood
IBD: seroprevalence and associated risk factors in major poultry r earing areas of Ethiopia
90.30%
Shiferaw et al.,2012
Debre-Zeit
Blood
Seroprevalence of infectious bursal disease in chickens managed under backyard production system
82.20%
Tesfaheywet and Getnet, 2012
Adea and Adami Tullu Gido Kombolcha
Blood
Seroprevalence of Newcastle disease and other infectious diseases in backyard chickens at markets
91.9% and 95.7%
Chaka et al.,2012
Eastern Ethiopia
Blood
Sero-Prevalence of IBD in Backyard chickens
83%
Tadesse and Jenbere, 2014
Mekele
Blood
Seroprevalence of infectious burs al disease in backyard chickens
45.05%
Sindu et al.,2015
East Showa zone
Blood
Epidemiology of Village
Chicken Diseases: Morbidityand Mortality: The Case of ND and IBD20.70%
Desalegn, 2015
Jimma Town and Bonga District
Blood
Seroprevalence and the Associated
Risk factors of IBD97.9% and 93.2%
Debebe,2016
Jigjiga and Harar Districts
Blood
Seroprevalence of IBD in Non-vaccinated Village Chicken
51.70%
Lemma et al., 2019
Table 1: Reported prevalence of IBD in Ethiopia (Source: synthesized by author).
Transmission of IBD Virus: Chickens are the only known avian species to develop clinical disease and distinct lesions when exposed to IBDV. The IBD transmit with horizontal way only, with healthy subjects being infected by the oral or respiratory pathway. Infected subjects excrete the virus in faces as early as 48 hours after infection, and may transmit the disease by contact over a sixteen-day period [80]. The most common mode of infection is through the oral route. Conjunctival and respiratory routes may also be involved [69]. Infected chickens begin to shed IBDV in faeces one day after infection and can transmit the disease for at least 14 days post infection [78]. The high persistence of the virus and its resistance to several disinfections and virucidal procedures may contribute to the rapid distribution of the virus [25]. IBDV may spread through contaminated equipment [34]. The disease is highly contagious, can also spread through the movement of poultry products, equipment, feed bags, vehicles and people and to a lesser extent, through aerosols of dust [18]. Transmission can also occur through airborne dissemination of virus-laden feathers or poultry house dust [46].
There is no evidence to suggest that IBDV is spread via transovarial transmission [19]. No specific vectors or reservoirs of IBDV have been established, but the virus has been isolated from mosquitos (Aedes vexans), rats, and lesser mealworms (Alphitobius diaperinus) [19]. Viable vvIBD virus was recovered after 2 days from the faeces of a dog that had been fed tissues from experimentally infected chickens, indicating that dogs may act as mechanical vectors for the virus [62]. There is no data that suggest IBDV is transmitted by wild birds, however direct or indirect transmission of the virus between wild birds and domestic chickens probably occurs [47]. In the absence of effective cleaning, disinfection and insect control; can increases the possibilities for transmission when they are scavenging of dead chickens, ingestion of contaminated water, or exposure of respiratory or conjunctiva membranes to contaminated poultry dust [60].
Morbidity and Mortality: Morbidity and mortality depend on the virulence of the challenged virus, the immune status and age of the infected birds and other factors affecting the pathogenicity of IBDV in full susceptible flocks, there is high morbidity rate usually approaching 100% [41]. Classical mortality ranges from zero to 30 %, but very virulent IBDVs strains can cause mortality of 70%-80% [52]. Infectious bursal disease is extremely contagious and in infected flocks, morbidity is high or with up to 100 % serological conversion, after infection, whilst mortality is variable [76]. In Europe, Africa and subsequently in Japan, high mortality rates of 50% to 60% in laying hens and 25% to 30% in broilers were observed. While in Ethiopia the mortality rate of the disease in different poultry houses ranges from 45-50%. The overall mortality rate was 49.89%. Broiler mortality was 56.09% while 25.08% for layer chickens [85]. These hyper virulent field strains caused up to 100 % mortality in Specific Pathogen Free (SPF) chickens [79]. Severity depends on the age and breed of the affected birds, the degree of passive immunity and the virulence of the strain of virus, and secondary infections associated with the immunosuppressive effects of the disease [79]. The most significant economic losses resulted from sub clinical infections of this form of IBDV infection greatly enhances the chicken’s susceptibility to sequel such as gangrenous dermatitis chicken anemia virus, inclusion body hepatitis, respiratory diseases and bacterial infections [45].
Risk Factors for IBD
The three main points where risks have been noticed are the breeding farms, the vaccine outlets and at the farm where the risk is twofold, i.e. biosecurity and vaccine handling [54]. The major risk factors however are at the farm and these include, but not limited to;
i. Few drinkers used for administering vaccine (thereby living out many birds not targeted for the vaccination).
ii. Presence of disinfectants in water that interferes with vaccine function
iii. Use of wrong vaccines (i.e. infectious bronchitis vaccines have been used instead of infectious bursal disease by uninformed cadre of farm workers/ managers) and less immunogenic IBD vaccines
iv. Use of improper diluents and vaccine adjuvants
Host range: Chicken is the only species of bird among the avian species known to be susceptible to IBDV where the virus induces clinical disease and causes IBD characteristic lesions [42]. Antibodies to IBDV have been detected in wild birds and several rare avian species including Antartic penguins, ducks, gulls, crows and falcons [19]. All breeds of chicken are affected but there is variation in severity of the disease between breeds [55]. White Leghorns exhibit the most severe disease and have the highest mortality rate [8]. Infectious Bursal Disease Virus (IBDV) is host specific. Although serologic evidence of natural infection with the virus has been reported in turkeys, ducks, guinea fowl and ostriches may be infected, clinical disease occurs solely in chickens [58]. It is strongly believed that the serotype IBDV 1 is highly host specific to chickens which develop IBD after infection by serotype 1 viruses. Reports have shown that serotype 2 of IBDV is more prevalent in many species of wild birds, with the natural host considered to be turkeys [61].
Infectious bursal disease virus has recently been isolated from a sparrow in China suggesting that wild birds could act as carriers [81]. The duck can also be an asymptomatic carrier of serotype 1 viruses [78]. There is no evidence that IBD virus can infect other animals, including humans [79].
Vaccination: Control of infectious bursal disease in chickens requires the application of sound biosecurity measures alongside effective vaccinations of chicks and parent flocks [49]. There are both live attenuated and inactivated vaccines for control of IBDV infections. Precise timing is crucial in administration of live vaccines to chicks due to interference of maternally derived antibodies on the performance of live vaccines [49]. On the other hand, high parental immunity is beneficial in protecting young chicks from field virus challenge during the critical first 2 weeks of life when the bursa is highly vulnerable to damage caused by IBDV [30].
Administration of inactivated vaccines to breeder hens induces long-standing and high levels of antibodies in the hatched chicks. But in some areas where very virulent IBD virus has caused significant losses the producers do not adopt inactivated vaccination. But intensive livevirus vaccination program is used in the hatched chicks from the unvaccinated breeder hens. Such chicks escape the strong risk of immunosuppressive form of the disease [84]. Inactivated vaccines do not replicate in the bird and are costly to produce and administer but have been found useful in administration to parent flocks prior to lay to provide passive immunity to offspring via maternally derived antibodies. The inactivated vaccines must have an antigenic content that is high enough to induce high immunity in parent flocks that can be passed to progeny at protective levels [67]. Usually, inactivated vaccines work best when administered in a prime-boost regimen, where attenuated live IBDV vaccines are first used for priming [49].
Live vaccines commonly used in chicks are suitable for mass vaccinations, do not require an adjuvant and can replicate in the bird to induce both humoral and cell mediated immunity [49]. One of the main side effects of the live vaccines is reversion to virulence resulting in disease and loss of production. Most conventional live vaccines are subjectively classified as mild, intermediate and intermediate plus or “hot” vaccines depending on the level of attenuation [65].
The mild vaccines do not neutralize high levels of maternally derived antibodies and in contrast some of the intermediate and most of the hot vaccines cause severe bursal lesions and could easily revert back to virulence [29]. Vaccinations have not been very successful in different parts of the world due to progressive changes in antigenicity and virulence of the virus and poor handling of the vaccines [54]. In view of this, however, vaccination still remains the single most important method of controlling IBDV in the field besides biosecurity. Other vaccines either being developed or already developed but not extensively used due to varied reasons include genetically engineered vaccines, subunit vaccines, viral vector vaccines and immune complex vaccines. Determining the timing of vaccination for chicks is highly dependent on the level of maternal antibody [49].
A wide variety of vaccine strains are commercially produced, mostly derived from classical virus strains, which do not all successfully protect against the vvIBD strains. “Hot” vaccines, which break through maternal antibody, are now in frequent use in countries which have vvIBD circulating, although their use risks causing bursal lesions, and as such may affect the response to other vaccinations or cause immunosuppression [49]. Failure of commercial vaccines to protect industrialized flocks in Ethiopia has prompted efforts to attenuate the local vvIBD strain to produce vaccines suitable for use within the country (Jenbreie, personal communication), although these will, again, be primarily intended for use on commercial units [37].
Biosecurity factor: An important characteristic of IBDV is its high stability in the environment, even after disinfection. Indeed, the virus can persist in installations for 54–122 days [6]. Due to the stable nature of the virus and the large amounts excreted following infection, it is practically impossible to remove all sources of infection once a rearing site has been contaminated. The dramatic impact of a very virulent IBD virus can be reduced by proper clean-up and disinfection between flocks, and that traffic (people, equipment and vehicles) onto the farm be controlled. The development and enforcement of a comprehensive biosecurity program is the most important factor in limiting losses by IBD due to IBD virus is very resistant and can survive for more than 100 days in a contaminated area. Phenolic and formaldehyde compounds have been shown to be effective for disinfection of contaminated premises [26].
Since the virus is very stable for months. It is largely excreted through feces hence contaminated litter, feed and water have to be burnt or buried deep under the lime cover [7]. There is evidence, however, that thorough cleaning and disinfection of houses between flocks and the practice of all-in all-out management reduces the challenge virus, afarm biosecurity measures reduce, but do not eliminate, the risk of infection and disease. As a United Kingdom leaflet [13] on poultry-farm biosecurity states: “Biosecurity means taking steps to ensure good hygiene practices are in place so that the risk of a disease occurring or spreading is minimized. Farm biosecurity practices cover a broad range of measures. These have been divided into three categories [68]:
¾Conceptual, including the choice of location for farms;
¾Structural, covering the physical facilities, such as netting to protect against entry of wild birds; and
¾Operational, covering the work procedures that farm staff and visitors are expected to follow. Field experience suggests that breakdowns in biosecurity can occur if attention is not paid to any one of these three elements.
Diagnosis
The clinical diagnosis of the acute forms of IBD is based on disease evolution of a mortality peak followed by recovery in five to seven days and relies on the observation of the symptoms and post-mortem examination of the pathognomonic lesions, in particular of the bursa of Fabricius [64]. Differential diagnoses, with respect to clinical signs, include avian coccidiosis, Newcastle disease in some visceral forms, stunting syndrome, inclusion body hepatitis, mycotoxicosis and infectious bronchitis. In subclinical and immunosuppressive forms of IBD, Marek’s disease and chicken anemia are also considered [42]; however, normally, these can easily be differentiated at post-mortem examination. The diseases like avian coccidiosis, Newcastle disease in some visceral forms, stunting syndrome, mycotoxicoses, and chicken infectious anemia and nephropathogenic forms of infectious bronchitis are the differential diagnosis for IBDV. In all acute cases, the presence of bursal lesions allows for a diagnosis of IBD [59]. In sub clinical cases, an atrophy of the bursa may be confused with other diseases such as Marek's disease or infectious anemia. A histological examination of the bursa will allow differentiation between these diseases [42].
Treatment; Control and Prevention
Control and Prevention: Infectious bursal disease virus is both highly contagious and very resistant to inactivation, which accounts for its persistent survival on poultry farms, despite disinfection [79]. Therefore, even with strict biosecurity programs (e.g. ‘down time’ between broods, all-in/all-out production, cleaning and disinfection of the premises and equipment), vaccination is especially important to reduce the incidence and impact of IBDV in the poultry industry [19]. Traditionally, breeder flocks are hyper immunized with live and killed vaccines in order to confer high titers of maternal antibodies to their progeny [79]. This passive immunity protects chicks against early immunosuppressive infections for 1 to 3 weeks; however, protection may be extended to 4 or 5 weeks by boosting the immunity in breeders with oil-adjuvanted vaccines [7].
Immunization of breeders is an important part of the IBDV control program. Antibodies produced by the hen are passed through the egg to the broiler chick. These maternal antibodies, if present in adequate levels, protect the chicks against sub clinical IBDV [33]. Live vaccines are administered to achieve active immunity but interference of Maternally Derived Antibody (MDA) is the crucial problem in determining a successful live IBDV vaccination schedule. Vaccinating chickens in the presence of high levels of maternally derived antibodies results in vaccine virus neutralization and no immunity [7]. Currently as reported by [70] in Mekele, Tigray, Ethiopia, determining the proper time for administration of live intermediate IBDV vaccine important than giving IBDV vaccine to chickens whose parents that have taken IBDV vaccine without determining Maternally Derived Antibodies (MDA) titer and age for vaccination [61]. Therefore, in order to have chickens protected from IBDV, it is crucial to determine the optimal timing for IBDV vaccine delivery [7].
Treatment: There is no specific therapy for the disease. Facilitate the access to water to prevent dehydration. As with every disease optimize climate and reduce stress to a minimum. Use of antibiotics can sometimes be advisable to limit the impact of secondary infections [85].
Economic Importance of Infectious Bursal Disease
Infectious bursal disease virus is worldwide in distribution and is an important virus in the poultry industry as it causes immune suppression and mortality in infected chickens [32]. The economic impact of IBD is influenced by the strain of the virus, susceptibility of flocks, intercurrent primary and secondary environmental and managemental factors, flock livability, weight gain, conversion and reproductive efficiency [68]. In addition to deaths, and immunosuppression, losses from IBD including depressed growth rate, feed conversion efficiency are recorded in affected broiler flocks [68].
Furthermore, the increase use of antibiotics and chemicals to fight secondary infections is a major concern of human health, if we consider the risks linked to the presence or residues in meat products, the release of residues into environment and increased antibiotic resistance [44]. The disease is a major set-back to productivity and profitability in the poultry industries of both developing and industrialized nationals. Direct losses linked to specific mortality depend on the dose and virulence of infecting IBDV strain, age and breed of the chicken and presence or absence of immunity [79]. Indirect economic impact of the disease, when quantified, is considerably significant [53]. It occurs due to virus induced immune-suppression and the interactions of IBDV and other viruses, bacteria or parasites [22]. Losses occur due to secondary infections, growth retardation and condemnation of carcasses at the slaughter houses [22]. Even if birds survive, the resulting immunosuppression and effect on egg production in layer birds is significant [50]. The virus does not affect man and has no direct public health significance [42].
Conclusion and Recommendation
Infectious bursal disease is a serious viral disease that has a great economic impact throughout poultry production areas. In Ethiopia infectious bursal disease is the main constraint to both commercial and backyard poultry production system. This disease is widely distributed in almost all part of the country imposes great losses on the economic development of the country. All birds are natural hosts of infectious bursal disease. The occurrence and distribution of this disease is not geographically bounded and studies reveal its prevalence up to 95.7% in the world. The most significant risk factors reviewed relating to this disease are age, breed, degree of immunity, strains of the virus, biosecurity and immunosuppression. Even if there are available studies regarding epidemiology of IBDV, an implementation of control program and lack of effective and specific vaccines are the main problem of this cosmopolitan disease.
Based on above conclusion, the following recommendations are forwarded.
¾Awareness on biosecurity approach to good sanitation on the poultry farm should be implemented by professionals.
¾Government should sponsor for control of Risk factors to reduce the magnitude of IBDV infection.
¾The current vaccine efficacy should be evaluated and Chickens should be vaccinated against Infectious Bursal Diseases (IBD).
Author Statements
Acknowledgements
Thanks, and praise to Almighty God, the Compassionate, and the Most Merciful for giving me health and blessing me with indomitable willpower, courage, strength and stamina to accomplish this arduous task. Next, I would like to express my sincere gratitude and deepest appreciation to my advisor Morka Dandecha who directs the path of progress. Words are inadequate to express my deep sense of indebtedness for his continuous constructive comments and tireless scientific guidance. I am extremely delighted in extending my thanks to Ambo University Guder Mamo Mezamir Campus School of Veterinary Medicine Department of Veterinary Science for facilitating internet access.
References
- Abdu PA, Mera UM, Saidu L. A study on chicken mortality in Zaria, Nigeria. Research National Workshop on Livestock and Veterinary Institute, Vom, Nigeria, August, 11-14th. 1992; 51-55.
- Aregitu M. Outbreak Investigation and Molecular Characterization of Infectious Bursal Disease Virus and Vaccine Immunogenesity Trial in Ethiopia (Doctoral dissertation). 2015.
- Asamenew T, Beshada T, Moti Y. Seroprevalence Study on Infectious Bursal Disease and Associated Risk Factors in Backyard Chicken Production in Sebeta Hawas District, Oromia, Ethiopia. European Journal of Applied Sciences. 2016; 8: 62-66.
- Aschalew Z, Esayas G, Teshale S, Gelagay A, Asegedech S, Bereket Z. Investigation on Infectious Bursal Disease Outbreak in Debre Zeit, Ethiopia. International Journal of Poultry Science. 2005; 4: 504-506.
- Ashenafi H. Survey of identification of major Diseases of local chickens in three selected agro climatic zones in central Ethiopia. DVM thesis, Addis Ababa University, Faculty of Veterinary Medicine, DebreZeit, Ethiopia. 2000; 1-60.
- Benton WJ, Cover MS, Rosenberger JK. Studies on the transmission of the infectious bursal agent of chickens. Avian Diseases. 1967; 11: 430-438.
- Besseboua O, Anad A, Benbarek H. Determination of optimal time of vaccination against infectious bursal disease virus (Gumboro) in Algeria. Onderstepoort Journal of Veterinary Research. 2015; 82: 887.
- Caston K. Infectious Bursal Disease Virus Segmented Double-Stranded RNA viruses; Structure and Molecular Biology. Caister Academic Press. 2008; 121-129.
- Chaka H, Goutard F, Bisschop SPR, Thompson PN. Seroprevalence of Newcastle disease and other infectious diseases in backyard chickens at markets in Eastern Shewa zone, Ethiopia. Poultry Science. 2012; 91: 862–869.
- Cosgrove AS. An apparently new disease of chickens: avian nephrosis. Avian diseases. 1962; 6: 385-389.
- CSA. FDRECSA: Federal Democratic Republic of Ethiopia Central Statistical Agency: Agricultural Sample Survey, Volume II. Report on Livestock and Livestock Characteristics, Addis Ababa, Ethiopia. 20-21.
- Debebe GA. Seroprevalence and the Associated Risk Factors of Infectious Bursal Disease in Chickens in Jimma Town and Bonga District of South West Ethiopia. A thesis submitted to the Faculty of Jimma University College of Veterinary Medicine and Agriculture, in partial fulfillment of the requirements for the Degree of Master of Science in Department of Clinical Study. 2016.
- Defra. Biosecurity and preventing disease. London, Department for Environment Food and Rural Affairs. 2006.
- Delmas B, Kibenge FSB, Leong FC, Mundt C, Vakharia VN, Wu JL. Birnaviridae. In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Academic Press: London. 2004; 561-569.
- Desalegn JM. Epidemiology of Village Chicken Diseases: A Longitudinal Study on the Magnitude and Determinants of Morbidity and Mortality. The Case of Newcastle and Infectious Bursal Disease. A thesis submitted to the Faculty of Addis Ababa University College of Veterinary Medicine and Agriculture, in partial fulfillment of the requirements for the Degree of Master of Science in Department of Clinical Study. 2015.
- Dessie T, Ogle B. Village poultry production system in the Central Highlands of Ethiopia. Tropical Animal Health and Production. 2001; 33: 521-537.
- Dinev I. Diseases of Poultry; A Colour Atlas, 4th Edition Eva Santé Anima. 2007; l549411125 9789549411126.
- Elankumaran S, Heckert R, Moura L. Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Diseases. 2002; 46: 169–76.
- Eterradossi N, Saif YM. Infectious bursal disease. In Y. M. Saif, A. M. Fadly, J. R. Glisson, L. R. McDougald, L. K. Nolan, and D. E. Swayne (eds.). Diseases of Poultry, 12th ed. Blackwell Publishing Professional: Ames. 2008; 185-208.
- Eterradossi N. Progress in the diagnosis and prophylaxis of infectious bursal disease in poultry. In Comprehensive Reports on Technical Items presented to the International Committee or to Regional Commissions. Office International des Epizooties, Paris. 1995; 75-82.
- Eterradossi N. Progress in the diagnosis and prophylaxis of infectious bursal disease in poultry. Journal of poultry science. 2000; 42: 36-64.
- Farooq M, Durrani FR, Imran N, Duran Z, Chaud N. Prevalence and Economic Losss Due to Infectious Bursal Disease in Broilers in Mirpur and Kotti Districts of Kashmir. International Journal of Poultry Science. 2003; 2: 267- 270.
- Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. Virus taxonomy: 8th report of the International Committee on Taxonomy of Viruses. Academic Press. 2005.
- Faragher JJ. Infectious bursal disease of chicken. Veterinary, Bull. 2001; 42: 361-369.
- Garriga D, Querol-Audí J, Abaitua F, Saugar I, Pous J, Verdaguer N, et al. The 2.6-Angstrom structure of infectious bursal disease virusderived T= 1 particles reveals new stabilizing elements of the virus capsid. Journal of virology. 2006; 80: 6895-6905.
- Gary DB, Richard DM. Review on Infectious bursal disease (Gumboro) in commercial broilers. http://edis.ifas.ufl.e College of Veterinary Medicine; UF/IFAS Extension, Gainesville, FL 32611. 2015.
- Gezali AA. Assesment of Poultry Production constraints and Evaluation of Different Concentrate on Production Performance and Parasitic Burden of Exotic Chickens In Kersa District of Jimma Zone (Doctoral dissertation, Jimma University). 2017.
- Hailu D, Melese B, Moti Y, Mekedes G. Seroprevalence of infectious bursal disease in backyard chickens. Veterinary Research Med well Journal. 2010; 4: 89-93.
- Hair-Bejo M, Ng MK, Ng HY. Day old vaccination against infectious bursal disease in Broiler chickens. Int. J. Poult. Sci. Asian Network for Scientific Information. 2004; 3:124-128.
- Hitchner SB. Immunization of adult hens against infectious bursal disease virus. Avian Diseases. 1976; 20: 611–613.
- Ingrao F, Rauw F, Lambrecht B, van den Berg T. Infectious Bursal Disease: A complex host-pathogen interaction. Development Company Immunology. 2013; 41: 429–438.
- Jackwood DJ, Sommer-Wagner SE, Stoute ST, Woolcock PR, Crossley BM, Hietala SK, et al. Characteristics of a Very Virulent Infectious Bursal Disease Virus from California, USA. Avian Diseases Digest. 2009; 4: 18-19.
- Jackwood DJ, Sommer SE. Molecular epidemiology of infectious bursal disease viruses: distribution and genetic analysis of newly emerging viruses in the United States. Avian Diseases. 2005; 49: 220-226.
- Jackwood D, Sommer-Wagner E. Detection and characterization of infectious bursal disease viruses in broilers at processing. Preventive Veterinary Medicine. 2010; 97: 45-50.
- Jacqueline MH. Characterization of Infectious Bursal Disease Viruses Isolated from Commercial Chickens. A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the Degree of Master of Science in Animal and Food Sciences. 2010; 1-148.
- Jayasundara JM, Walkden Brown SW, Katz ME, Islam AF, Renz KG, McNally J. et al. Pathogenicity, tissue distribution, shedding and environmental detection of two strains of IBDV following infection of chickens at 0 and 14 days of age. Avian Pathology. 2016; 1–14.
- Judy MB. The epidemiology and ecology of infectious diseases in Ethiopian village chickens and the role of co-infection in infection risk. A thesis submitted in accordance with the requirements of the University of Liverpool, in partial fulfillment of the requirements for the degree of Doctor in Philosophy. 2014.
- Kasanga CJ, Yamaguchi T, Wambura PN, Munangandu HM, Ohya K, Fukushi H. Detection of infectious bursal disease virus (IBDV) genome in free-living pigeon and guinea fowl in Africa suggests involvement of wild birds in the epidemiology of IBDV. Virus Genes. 2008; 36: 521–529.
- Lasher HN, Davis VS. History of infectious bursal disease in the U.S.A, The first two decades. Avian Diseases. 1997; 41: 11-19.
- Lemma F, Zeryehun T, Kebede A. Seroprevalence of Infectious Bursal Disease in Non-vaccinated Village Chicken in Jigjiga and Harar Districts, Eastern Ethiopia. J Vet Sci Technol. 2019; 10: 572.
- Lukert PD, Saif YM. Infectious bursal disease. In: Diseases of Poultry, 11th edition, Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR and Swayne DE (eds), Iowa State Press, Ames, Iowa. 2003; 161–179.
- Lukert PD, Saif YM. Infectious bursal disease. In: Diseases of poultry, Tenth Edition, Calnek, B.W, edition. IOWA state university press, Ames, IOWA, USA. 2004; 721-738.
- Mahgoub HA. An overview of infectious bursal disease. Archives Virology. 2012; 157: 2047-2057.
- Marian Burros. Eating well: Shopping for Antibiotic free Meat. 2001.
- Mazengia H, Tilahun SB, Negash T. Incidence of infectious bursal disease in village chickens in two districts of Amhara Region, Northwest Ethiopia. Journal Livestock Research Rural Development. 2009; 21: 12.
- Mazengia H. Review on major viral diseases of chickens reported in Ethiopia. Journal of Infectious Disease and Immunology. 2010; 4: 34-39.
- Minalu T, Tewodros F, Bemrew A. Infectious Bursal Disease (GUMBORO Disease) in Chickens. British Journal of Poultry Sciences. 2015; 4: 22-28.
- Mohamed AM, Elzanaty ESK, Bakhit BM, Safrat MM. Genetic Characterisation of infectious bursal disease virus Associated with Gumboro Outbreaks in Commercial Broilers from Asyut Province, Egypt. Veterinary Science. 2014; 2014: 916412.
- Müller H, Mundt E, Eterradossi N, Islam MR. Current status of vaccines against infectious bursal disease. Avian Pathology. 2012; 41: 133-139.
- Muller H, Islam MR, Raue R. Research on Infectious Bursal Disease the Past, the Present and the Future. Veterinary Microbiology. 2003; 97: 153–165.
- Mulugeta A, Tebkew A. Evaluation of indigenous chicken productivity by using a questioner survey, in selected Chagni town, Awi-administrative zone, Amhara Region, Ethiopia. World Journal of Agricultural Sciences. 2013; 1: 26-35.
- Murphy FA, Gibbis EPJ, Horzinek MC, Studdert MJ. Birna viridae in: Veterinary Virology, third edition. Academic press, San Diego, California, USA. Chapter. 1999; 25: 405-409.
- Musa LW, Saidu L, Abakaka EES. Economic impact of recurrent outbreak of Gumboro disease in a commercial poultry farm Kano, Nigeria, Asia. Journal of Poultry science. 2012; 89: 525-565.
- Mutinda WU, Nyaga PN, Bebora LC, Mbuthia PG. Vaccination against Infectious bursal disease fails to yield protective antibody titers in chickens in Kwale Kenya. The Kenya Veterinarian. 2016; 39: 33-37.
- Mutinda WU, Nyaga PN, Mbuthia PG, Bebora HV, Mucheni G. Risk factors associated with infectious bursal disease vaccination failures in broiler farms in Kenya. Tropical Animal Health Production. 2013; 46: 603-608.
- OIE. World Animal Health Information Database (WAHID) Interface. 2013.
- Office of International des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Infectious Bursal Disease: Chapter. 2012; 12: 549-565.
- Office International des Epizooties. Infectious bursal disease. 2008; 549–565.
- Office Internationals des Epizootics. Manual of diagnostic tests and vaccines for Terrestrial Animals.5th edition, Infectious Bursal Disease (Gumboro disease). 2004.
- Okoyo JO, Uzoukwu M. Infectious bursal disease among chickens. Avian Disease. 2005; 25: 1034-103.
- Okwor EC, Eze DC, Okonkwo K. Serum Antibody Levels against Infectious bursal disease virus in Nigerian Village Chickens. Pakistan Veteterinary Journal. 2011; 32: 286-287.
- Pages-Mante A, Torrents D, Maldonado J, Saubi N. Dogs as potential carriers of infectious bursal disease virus. Avian Pathology. 2004; 33: 205–209.
- Quinn PJ, Carter ME, Donnely WJ, Leonard FC, Markey BK. Veterinary microbiology and microbial disease.1st edition Corn wall: Blackwell publishing press. 2002; 375.
- Rajaonarison JJ, Rakotonindrina SM, Rakotondramary EK, Razafimanjary S. Gumboro Disease (Infectious bursitis) in Madagascar. Revue d’élevage et de medicine vétérinaire des pays tropicaux. 2006; 47: 15-17.
- Rautenschlein S, Kraemer C, Vanmarcke J, Montiel E. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Diseases. 2005; 49: 231-7.
- Rautenschlein S, Alkiel TN. Infectious bursal disease virus in poultry: current status and future prospects. Veterinary Medicine. 2016; 2016: 9-18.
- Rosenberger JK, Cloud SS, Metz A. Use of infectious bursal disease virus variant vaccines in broiler breeders. In: Proceedings of 36th Western Poultry Disease Conference. Davis, C.A. 1987; 105-107.
- Shane S. The poultry industry handbook. Singapore. American Soybean Association Southeast Asia. 1997.
- Sharma JM, Kim IJ, Rautenschlein S, Yeh HY. Infectious bursal disease virus in chickens: pathogenesis and immunosuppression. Developmental and Comparative Immunology. 2000; 24: 223-235.
- Shiferaw J, Gelagay A, Esayas G, Fekadu K, Stacey EL, Haileleul N. Infectious bursal disease: seroprevalence and associated risk factors in major poultry rearing areas of Ethiopia. Tropical Anim Health Production. 2012; 45: 75-79.
- Sinidu Z, Yisehak T Haftay A, Nesibu A. Seroprevalence of infectious bursal disease in backyard chickens. African Journal of Biotechnology. 2015; 14: 434-437.
- Solomon W, Abebe W. Infectious bursal disease (Gumboro disease) case report of Andasa poultry farm Amahara region. Ethiopian Veterinary Journal. 2007; 11: 151.
- Tadelle D, Million T, Alemu Y, Peters K. Village chicken production systems in Ethiopia: 2. Use patterns and performance valuation and chicken products and socio-economic functions of chicken. Livestock Research for Rural Development. 2003c; 15.
- Tadesse B, Jenberie S. Sero-Prevalence of Infectious Bursal Disease in Backyard chickens at Selected Woredas of Eastern Ethiopia. Journal of Biology, Agriculture and Healthcare. 2014; 4: 2224-3208.
- Tesfaheywet Z, Getnet F. Seroprevalence of infectious bursal disease in chickens managed under backyard production system in Central Oromia, Ethiopia. African Journal of Microbiology Research. 2012; 6: 6736-6741.
- Tsegaye K, Mersha Ch. Review on the Incidence and Pathology of Infectious Bursal Disease. British Journal of Poultry Sciences. 2014; 3: 68-77
- USAID (United states Agency for International Development). Livestock Market Development Project. Addis Ababa, Ethiopia. 2013.
- Van den Berg TP, Eterradossi N, Toquin D, Meulemans G. Infectious bursal disease (Gumboro disease). Scientific and Technical Review International Office of Epizootics. 2004; 19: 509-543.
- Van den Berg T. Birnaviridae. Poultry Diseases, 6th Edition (Pattison, M., McMullin P., Bradbury J., Alexander D.) Saunders, Elsevier. 2007; 491.
- Vindevogel H, Gouffaux M, Meulemans G, Duchatel JP, Halen P. Maladie de Gumboro: distribution et persistance du vims chez le poussin inoculé. Études sur la transmission de la maladie. Avian Pathol. 1976; 5: 31-38.
- Wang G, Qian F, Ping L. The epidemic characteristics and comprehensive prevention control of infectious bursal disease in our country. China Diseases Control. 2007; 143: 25-27.
- Wang G, Qian F, Ping L. The epidemic characteristics and comprehensive prevention control of infectious bursal disease in our country. China Disease Control. 2009; 143: 25-27.
- Woldemariam S, Wossene. Infectious bursal disease (Gumboro disease): Case report at Andassa poultry farm, Amhara region. Veterinary Journal. 2007; 11: 141-150.
- Wu CC, Rubinelli P, Lin TL. Molecular detection and differentiation of infectious bursal disease virus. Avian Diseases. 2007; 5: 512-526.
- Zeleke A, Gelaye E, Sori T, Ayelet G, Sirak A, Zekarias B. Investigation on infectious bursal disease outbreak in Debre Zeit, Ethiopia. International Journal of Poultry Science. 2005a; 4: 504-506.