
Research Article
J Bacteriol Mycol. 2024; 11(2): 1223.
Comparative Analysis of Xpert MTB/RIF and Xpert Ultra Cartridges for Rapid Detection of Tuberculosis: A Retrospective Point-of-Care Testing
Sangeetha Subaramani1; Maria Joes1; Vijayalakshmi Prakash1; Usharani Brammacharry2; Venkateswari Ramachandra3; Revathi Mani Blasundaram4; Gunavathy Pradhabane5; Anbazhagi Muthukumar6; Sriramkumar Srikrishnan Rajendran7; Muthuraj Muthaiah1*
1Department of Microbiology, State TB Training and Demonstration Centre, Intermediate Reference Laboratory, Government Hospital for Chest Diseases, Puducherry, India
2Department of Medical Biochemistry, Institute of Basic Medical Sciences, University of Madras, Tamil Nadu, India
3Department of Biochemistry, Queen Mary’s College, Madras, Tamil Nadu, India
4Department of Biotechnology, Indira Gandhi College of Arts and Science, Indira Nagar, Puducherry, India
5Department of Genetics, Institute of Basic Medical Sciences, University of Madras, Tamil Nadu, India
6Department of Environmental Science, Central University, Kasaragod, Kerala, India
7Centre for Global Health Research, Saveetha Institute of Medical and Technical Sciences, Saveetha Nagar, Thandalam, Chennai-602105, India
*Corresponding author: Muthuraj Muthaiah, Department of Microbiology, State TB Training and Demonstration Centre, Intermediate Reference Laboratory, Government Hospital for Chest Diseases, Puducherry, India. Tel: +91 9944737597 Email: drmuthurajm@gmail.com
Received: November 01, 2024; Accepted: November 20, 2024 Published: November 27, 2024
Abstract
The World Health Organization (WHO) has endorsed the next-generation Xpert MTB/RIF Ultra cartridge. High-burden countries are gradually transitioning from the older Xpert MTB/RIF (Xpert) cartridge to Ultra as the initial diagnostic test for both pulmonary and extrapulmonary Tuberculosis (TB). This study aims to assess the diagnostic accuracy of Ultra for detecting TB in both forms. From January to September 2024, presumptive TB patients visiting TB Screening and Treatment centres in Puducherry for routine Chest X-Rays (CXR) and conventional Xpert testing were enrolled. A total of 2,302 cases were included, comprised of 418 extrapulmonary and 1,884 pulmonary tuberculosis cases. Single respiratory specimens from symptomatic suspects accessing healthcare services were tested using fluorescence microscopy, culture, Xpert, and Ultra. The liquid culture method (MGIT) was used as the composite reference standard. The results indicate that Xpert Ultra has an overall sensitivity of 100% and a specificity of 96.96%. In comparison, Xpert showed a sensitivity of 90.88% and a specificity of 99.65%, while fluorescence microscopy had a sensitivity of 54.87% and a specificity of 100%. Consequently, Xpert Ultra emerges as a breakthrough in tuberculosis diagnosis. Its high sensitivity and specificity can potentially supplement and replace conventional diagnostic methods, setting a new standard for detecting pulmonary and extrapulmonary Tuberculosis.
Keywords: Mycobacterium tuberculosis, Rifampicin resistant, Sensitivity, Specificity, Accuracy
Introduction
Tuberculosis (TB) remains the deadliest infectious disease caused by a single agent among all communicable diseases [5]. Globally, an estimated 10.6 million people continue to fall ill with TB every year, with over 1.3 million deaths occurring annually [1]. India accounts for about 25% of the global TB burden, with an estimated TB incidence of 27.8 lakh in 2023, slightly increased from the previous year's estimate of 27.4 lakh in 2022 [2]. In 2023, the estimated percentage of new TB cases with MDR/RR-TB decreased to 3.3%, while the percentage of previously treated cases with MDR/RR-TB dropped to 17%. The countries with the highest number of MDR/ RR-TB cases in 2023 were India (26% of global cases), the Russian Federation (8.5% of global cases), and Pakistan (7.9% of global cases). Urgent action is needed to eliminate the global TB epidemic by 2030, a goal adopted by all Member States of the United Nations (UN) and the World Health Organization [3]. The development of rapid and accurate diagnostic tests for tuberculosis (TB), which decreases the time of treatment initiation, is an important strategy to control the TB epidemic. A delay in diagnosing tuberculosis (TB) can lead to prolonged infectivity, delayed treatment, and increased disease severity. The delay in diagnosing and treating multidrug-resistant tuberculosis is associated with poor treatment outcomes in patients. It is essential to quickly diagnose Mycobacterium tuberculosis complex from clinical specimens in order to effectively treat TB patients and reduce the transmission rate. While acid-fast bacilli microscopy (AFB microscopy) is a fast and simple diagnostic method, it has low sensitivity, particularly for extrapulmonary specimens. Additionally, mycobacterial culture, which is considered the gold standard, takes several weeks to confirm the diagnosis [4].
The World Health Organization endorsed the Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) assay, used to diagnose tuberculosis and rapidly detects rifampicin resistance. It is a fully automated hemi-nested real-time PCR point-of-care assay that can detect both the presence of the M.tuberculosis complex and rifampicin resistance (RIF-R) associated mutations in the rpoB gene within 2 hours. Although it rapidly became the front-line test for diagnosing tuberculosis in high-burden countries worldwide, Xpert exhibited suboptimal sensitivity in paucibacillary cases, and there were concerns about its ability to detect certain rifampicin silent mutations [5].
The Xpert has a Limit of Detection (LOD) of approximately 113 CFU/ml (Colony Forming Unit per ml), which is less sensitive than culture, which has a LOD of between 1 and 10 CFU/mL [6]. In 2017, new and improved Xpert Ultra cartridges were introduced by Cepheid, Sunnyvale, CA, USA, to address limitations with the existing G4 cartridges and enhance diagnostic efficiency [7]. The Xpert Ultra uses the same diagnostic platform as Xpert but incorporates several changes. These changes include fully nested nucleic acid amplification, a larger polymerase chain reaction chamber, incorporation of two multicopy polymerase chain reaction amplification targets (IS6110 and IS1081), and the use of melt curve analysis to detect RIF resistance [8].
These modifications have improved the limit of detection from 113 bacilli/mL in the G4 cartridge to 16 bacilli/mL in the Ultra cartridge, thereby increasing the sensitivity of the Ultra cartridge to 78.9% in smear-negative samples, which is higher than Xpert (66.1%) [9]. Additionally, the Ultra cartridge provides "trace" interpretations when the samples are positive without rpoB gene signals. However, this has led to a loss of specificity, resulting in almost a twofold increase in false positives in patients with no prior history of TB. Additionally, the overall turnaround time decreased by 77 minutes for the amplification of MTB genetic materials [10].
Materials and Methods
Study Setting and Design
A retrospective study was conducted at the Government Hospital for Chest Disease in Puducherry, South India, from January 2024 to September 2024. The study included pulmonary and extrapulmonary tuberculosis patients with confirmed drug-resistant tuberculosis from Puducherry state between January 2020 and December 2023. Patients were instructed to collect samples in a pre-labelled, sterile 50ml widemouthed falcon tube before starting treatment. The samples were then packed in a standard three-pack container with an ice pack and sent to the Intermediate Reference Laboratory along with an examination form. The laboratory analyzed the samples using fluorescence microscopy and phenotypic and genotypic diagnostics. A total of 2302 TB suspects from public sector tertiary healthcare facilities, three major civil hospitals, and nine medical colleges in Puducherry state were enrolled in the study. Patients with incomplete data and undocumented methods of diagnosis were excluded.
Smear Microscopy by Light-Emitting Diode Fluorescent Microscopy
Clean and grease-free slides were used and completely covered with Auramine O solution (Sigma-Aldrich, Machelen, Belgium). After 20 minutes, the slides were washed and decolorized with a 0.5% acid alcohol solution for 3 minutes, followed by counter-staining with 0.5% potassium permanganate for 1 minute. The stained smears were examined under a LED-FM (Primo Star iLED, Carl Zeiss, Gottingen, Germany) with 400X magnification, and 40 fields were examined. The results were reported for the presence or absence of AFB using the World Health Organization/International Union Against Tuberculosis and Lung Disease scale, with a positive result corresponding to ≥ 1 AFB per 20x for screening and 40x for confirmation [11].
Lymph Nodes and Tissue Samples Processing for Xpert MTB/RIF and Xpert Ultra Assay
The lymph nodes and other tissue samples were cut into small pieces using clean and sterile forceps and dissection knives in a sterile mortar. Approximately 2 mL of sterile Phosphate Buffer (PBS) was added to the container with the dissected tissue pieces. The mixture was ground with a mortar and pestle until it formed a consistent solution. Subsequently, approximately 0.7 mL of the homogenized tissue sample was transferred to a sterile conical screw-capped tube using a transfer pipette. Following this, a double volume of Xpert MTB/RIF Sample Reagent (1.4 mL) was added to the 0.7 mL of homogenized tissue, and the solution was vigorously shaken for at least 10 seconds using a vortex. The suspension was then incubated for 10 minutes at room temperature and shaken vigorously for at least 10 seconds using a vortex. The processed sample was incubated for another 5 minutes at room temperature, and then 2 mL of it was transferred to the Xpert MTB/RIF cartridge using a fresh sterile transfer pipette. It was ensured that the correct laboratory number was recorded, matching the cartridge and sputum cup numbers. The prelabelled barcode was then scanned on the cartridges after switching on the system attached to the Xpert instrument. Finally, following the manufacturer's instructions, the cartridge was loaded into the Xpert instrument. The green light stopped blinking after clicking to start the test, and the test began. After completion of the test, the light turned off, and the results were printed automatically. It was necessary to wait until the system released the door lock at the end of the run, then open the module door and remove the cartridge. The used cartridges were disposed of in the biohazard waste container [12,13].
Processing of CSF and other liquid Samples for Xpert MTB/RIF and Xpert Ultra Assay
If the volume of Cerebrospinal Fluid (CSF) or any liquid sample is less than 2 mL, add an equal volume of Xpert MTB/RIF reagent to the CSF sample. Then, about 2 mL of the sample mixture was transferred directly to the Xpert MTB/RIF cartridge. After that, load the CSF sample cartridge into the Xpert instrument following the manufacturer's instructions. On the other hand, if the sample volume exceeds 2 mL, transfer all sample content to a sterile conical centrifuge tube. Centrifuge the tube for 15 minutes at 4000 rpm. After centrifuging, carefully discard the supernatant into a discard bin containing 5% phenol or other mycobacterial disinfectants. Then, 2 mL of Xpert MTB/RIF sample reagent was added to the deposit using a fresh sterile transfer pipette. Transfer 2 mL of the concentrated CSF sample to the Xpert MTB/RIF cartridge. Record the correct laboratory number, matching the cartridge and sputum cup numbers. Scan the pre-labeled barcode in the cartridges after switching on the system attached to the Xpert instrument. Finally, the cartridge is loaded into the Xpert instrument following the manufacturer's instructions. The test starts, and the green light stops blinking after clicking to start the test. Once the test is finished, the light turns off. Results are automatically printed once the run is completed. Wait until the system releases the door lock at the end of the run, then open the module door and remove the cartridge. Dispose of the used cartridges in the biohazard waste container [14].
Liquid Culture and Identification
The sputum sample was decontaminated using the N-acetyl-Lcysteine and sodium hydroxide (NALC/NaOH) method with a final NaOH concentration of 1%. An equal volume of standard NALC/ NaOH solution was added to the specimen and incubated for 15 minutes. After centrifugation for 15 minutes at 3000 x g, the sediment was re-suspended in 1 mL of sterile phosphate-buffered saline. 500 μL of the resulting pellets was inoculated into the MGIT tubes. In each run, M. tuberculosis strain H37Rv was used as a positive control. MGIT tubes were inoculated with sterile phosphate-buffered saline for the negative control [15]. Differentiation of the M. tuberculosis complex from nontuberculous mycobacteria was performed using the SD BIOLINE MPT64 TB Ag test (Standard Diagnostics, Yongin, South Korea) [16].
Statistical Analysis
We performed all statistical analyses using MedCalc software (version 22.026) [17]. We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of different assays at a 95% Confidence Interval (CI) against mycobacterial culture as well as a Composite microbiological Reference Standard (CRS). The CRS included the bacteriological confirmation tests: Ultra, Xpert G4, culture, and AFB microscopy. We used the Chi-square test to calculate the p-value, and the results were considered statistically significant if the p-value < 0.05. Additionally, we calculated the Kappa (k) value for assay agreement, categorized as follows: ≤0; no agreement, 0.1–0.4; fair agreement, 0.41–0.6; moderate agreement, 0.61–0.8; substantial agreement, 0.81–1.0; complete agreement.
Results
Among 2302 specimens, 1884(81.84%) were pulmonary and 418(18.16%) were epTB samples. Out of 2302 specimens, 19(0.8%) and 6(0.3%) were invalid and had no results, respectively. Of 1,884 pulmonary TB samples, 493 (26.17%) tested positive. In contrast, 61 (14.59%) of the 418 epTB samples were positive. Out of 2277 specimens, 554 (24.3 %), 498 (21.8 %), 500 (22.0 %), and 304 (13.4 %) tested positive by Ultra, Xpert G4, Liquid culture, and FM microscopy, respectively. Of 554 tested positive, Ultra and Xpert showed 100 % concordance for RIF resistant 40.4 % (201/498). Out of the 554 individuals who tested positive, 304 were tested positive by all four technologies, while 188 were detected by Ultra, Xpert G4, and MGIT culture. Additionally, 6 were found by both Ultra and Xpert G4, and 8 by Ultra and MGIT culture (Figure 1).
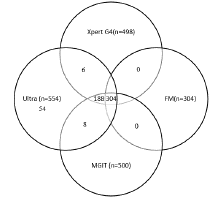
Figure 1: Positive Mycobacterial tests in individuals with at least one
confirmatory test for Tuberculosis.
554 positive mycobacterial tests (554 positive by Xpert Ultra, 498
by Xpert G4,304 by Fluorescence Microscopy, and 500 by MGIT)
MGIT=Mycobacteria Growth Indicator Tube. Ultra: Xpert MTB/RIF Ultra.
Xpert G4: Xpert MTB/RIF.FM: Fluorescence Microscopy.
In comparison to culture results, the diagnostic performance of the tests was as follows: Ultra had a sensitivity of 100% and specificity of 96.96%, Xpert had a sensitivity of 90.88% and specificity of 99.65%, and AFB microscopy had a sensitivity of 54.87% and specificity of 100%. When 'trace' cases were excluded from the analysis, the specificity of Ultra increased to 99.54%. The overall diagnostic accuracy for Ultra with 'trace,' Ultra without 'trace,' Xpert, and AFB microscopy was 97.63%, 99.64%, 97.54%, and 89.02%, respectively. Overall, Ultra demonstrated excellent agreement with culture (k = 0.93). When excluding 'trace' cases, Ultra showed even stronger agreement (k = 0.99). Xpert had good agreement (k = 0.93), while AFB microscopy showed moderate agreement (k = 0.65) with culture results (Table 1)
Test Methods
MTB Detection
MGIT (n-500)
Sensitivity (%)
Specificity (%)
PPV (%)
NPV (%)
Accuracy (%)
Kappa
Positive
Negative
(95 % CI)
(95 % CI)
(95 % CI)
(95 % CI)
(95 % CI)
Ultra (n-554)
Detected
500
54
100 (99.26-100)
96.96 (96.05-97.71)
90.25 (87.69-92.33)
100 (99.79-100)
97.63 (96.92-98.21)
0.93 (0.92-0.95)
Not Detected
0
1723
Ultra (Excluding trace) n-500
Detected
492
8
100 (99.25-100)
99.54 (99.09-99.80)
98.4 (96.86-99.19)
100 (99.79-100)
99.64 (99.29-99.84)
0.99 (0.98-0.99)
Not Detected
0
1723
Xpert G4(n-498)
Detected
498
6
90.88 (88.15-93.15)
99.65 (99.25-99.87)
98.81 (97.39-99.46)
97.18 (96.36-97.82)
97.54 (96.82-98.14)
0.93 (0.91-0.95)
Not Detected
50
1723
AFB - FM (n-304)
Detected
304
0
54.87 (50.62-59.07)
100 (99.79-100)
100 (98.79-100)
87.33 (86.28-88.31)
89.02 (87.66-90.28)
0.65 (0.61-0.69)
Not Detected
250
1723
Table 1: Diagnostic performance of different assays compared to MGIT culture.
Out of 554 ultra-positive cases tested, 54 were reported as "Not Detected" by the Xpert assay (Figure 2). The "trace" result accounted for 2.37% (54/2277) of the total specimens and 9.75% (54/554) of the ultra-positive cases that were negative by other tests. The patients with a "trace" result had no prior history of Tuberculosis (TB), and the final diagnosis was based on a correlation with clinical TB symptoms. We attempted to follow up on the "trace" positive cases to assess their clinical diagnosis and treatment status. During the study period, we successfully followed up with 47 out of 54 patients, which is 87.03%. After correlating the clinical signs and symptoms of extra pulmonary TB (EPTB) with the "trace" results, 29 patients (53.7%) were ultimately diagnosed with TB and began anti-TB treatment immediately.
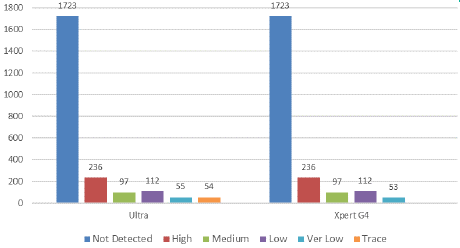
Figure 2: Comparison between Ultra and Xpert G4 assay depending
on the range of PCR cycle threshold (Ct) value [High: (Ct value: <16),
Medium: (Ct value: 16–22), low (Ct value: 22–28), Very low (Ct value: >28),
Trace (due to the presence of only IS6110 and/or IS1081 molecular signals
in the absence of at least 3 of the probe probes)].
Discussion
The main strength of our study lies in the prospective inclusion of extra pulmonary specimens from a clinical setting, enabling us to evaluate diagnostic accuracy and feasibility in real-world applications. Another advantage is our comparison of the Ultra results with culture, which serves as the reference standard. In this study, we compared the performance of the new Xpert Ultra assay with the existing Xpert G4 assay and auramine smear microscopy for detecting Tuberculosis in clinical specimens from patients with both pulmonary and extra pulmonary Tuberculosis. These tests are utilized as rapid initial diagnostic tools for individuals suspected of having Tuberculosis. Our results indicate that the new Xpert Ultra assay exhibits the highest sensitivity at 100% for detecting TB in both pulmonary and extra pulmonary specimens. The Xpert G4 assay follows with a sensitivity of 90.88%, while auramine smear microscopy shows the lowest sensitivity at 54.87%. The findings align with recent reports indicating that Xpert Ultra has superior sensitivity for detecting Tuberculosis (TB) cases [4] [4]. In our study, the sensitivity of Xpert Ultra was 100.0%, which is higher than the 90.88% sensitivity of the standard Xpert. However, this increased sensitivity came at the cost of reduced specificity, with values of 96.96% for Ultra compared to 99.65% for Xpert. This reduction is attributed to the additional 'trace' category (9%), a phenomenon also observed in other studies [18,4].
The existing Xpert G4 had some limitations, especially in situations where the bacillary load was low. To enhance the diagnostic efficiency and utility of the previous assay, Cepheid developed the latest version, the Xpert Ultra cartridge, which is compatible with the pre-existing GeneXpert platform [19]. The new cartridge incorporates two significant improvements. First, the DNA amplification process has been optimized by increasing the chamber volume from 25μl to 50μl. Second, it introduces new insertion sequences, IS1081 and IS6110. As a result of these changes, the detection limit for Mycobacterium Tuberculosis (MTB) has drastically improved, rising from 131 bacilli/ml of sputum to just 16 bacilli/ml. Additionally, the overall turnaround time for amplifying MTB genetic material has been reduced by 77 minutes. In Xpert Ultra, semi-quantitative results are classified as high, medium, low, and very low, along with a new category labelled "Trace." The Rifampicin (RIF) resistance status is reported as detected, not detected, or intermediate [20].
Overall, Xpert Ultra demonstrated a 10% higher sensitivity than Xpert MTB/RIF in pulmonary and extra pulmonary tuberculosis (epTB) samples, although this difference was not statistically significant. Additionally, a minority of the samples (9%) showed trace readouts. This rate is lower than the 10% trace readout reported by Berhanu et al. [21], but higher than the 3% found by Esmail et al. [9] in South Africa. With progressive improvements in assay quality, Xpert Ultra has enhanced detection rates while maintaining similar sensitivity patterns, all within a shorter turnaround time. This enables quicker initiation of anti-tubercular therapy, effectively breaking the chain of transmission. The new semi-quantitative category in Xpert Ultra, known as MTB Trace, has increased sensitivity by 5% but decreased specificity by 3.2% compared to the previous Xpert version. According to WHO recommendations, the Ultra test provides results for all types of smear-positive and smear-negative respiratory specimens, similar to the earlier version of the Xpert kit. In a recent study by Mishra et al. [22], Trace results were predominantly seen in patients who had previously been treated for tuberculosis, and whose culture results were mostly negative. This resulted in suboptimal specificity. However, specificity improved by 5-15% when all such results were re-categorized as MTB not detected.
The current WHO guidelines indicate that trace readouts should suggest Tuberculosis treatment in cases of paucibacillary disease. In other situations, however, repeat testing should be conducted [20]. In regions with a high burden of tuberculosis, interpreting 'trace' results can be challenging, especially for patients with a prior history of Tuberculosis, which raises the risk of over diagnosis [19]. The clinical significance of trace readouts remains ambiguous, which can affect treatment decisions. As a result, a Tuberculosis diagnosis might be overlooked, or patients could receive incorrect prescriptions for potentially harmful treatments. Our study emphasizes that tracepositive results in individuals with a prior history of Tuberculosis should be carefully evaluated before starting Tuberculosis treatment. Future prospective studies involving patients with trace results are necessary to offer better guidance on how to optimally manage these patients in various scenarios.
Conclusion
Our study demonstrates that the Ultra test can diagnose pulmonary and extra pulmonary tuberculosis more efficiently than the Xpert G4 test, although it shows a slight reduction in specificity compared to culture methods. Therefore, we recommend using the Ultra assay as a preliminary diagnostic tool for extra pulmonary tuberculosis wherever possible. However, results categorized as 'trace' should be interpreted cautiously, considering the patient's clinical signs, symptoms, and histopathological findings before initiating treatment. In patients with no prior history of tuberculosis, a 'trace' result from extra pulmonary specimens should be regarded as a true positive. Further studies with a larger sample size across multiple centres are needed to investigate the frequency and clinical significance of falsepositive results using the Ultra test.
Author Statements
Acknowledgments
The authors would like to acknowledge the staff of the Intermediary Reference Laboratory and State TB cell (NTEP) for their skilful technical assistance.
Author Contributions
SS, VP and MJ--prepared manuscript; BU and RV--prepared figures; PG and BRM--prepared tables; AM, SSR and MM--statistical analysis. All authors reviewed the manuscript.
Data Availability Statement
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India. Permission is granted to the corresponding author to access the data through login credentials. The datasets are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Global tuberculosis report 2023. Geneva, Switzerland: World Health Organization. 2023.
- India TB reports. Central TB Division Ministry of Health and Family Welfare. 2023.
- Global Tuberculosis Programme. Global TB Strategy with ambitious targets. 2015.
- Nasrin R, Uddin MKM, Kabir SN, Rahman T, Biswas S, Hossain A, et al. Xpert MTB/RIF Ultra for the rapid diagnosis of extrapulmonary tuberculosis in a clinical setting of high tuberculosis prevalence country and interpretation of ‘trace’ results. Tuberculosis. 2024; 145: 102478.
- Saavedra B, Mambuque E, Nguenha D, Gomes N, Munguambe S, Garcia JI, et al. Performance of Xpert MTB/RIF Ultra for tuberculosis diagnosis in the context of passive and active case finding. Eur Respir J. 2021; 58: 2100257.
- Mansfield M, McLaughlin AM, Roycroft E, Montgomery L, Keane J, Fitzgibbon MM, et al. Diagnostic Performance of Xpert MTB/RIF Ultra Compared with Predecessor Test, Xpert MTB/RIF, in a Low TB Incidence Setting: a Retrospective Service Evaluation. Microbiol Spectr. 2022; 10: 02345-21.
- Donovan J, Cresswell FV, Thuong NTT, Boulware DR, Thwaites GE, Bahr NC. Xpert MTB/RIF Ultra for the Diagnosis of Tuberculous Meningitis: A Small Step Forward. Clin Infect Dis. 2022; 71: 2002-2005.
- Wang G, Wang S, Yang X, Sun Q, Jiang G, Huang M, et al. Accuracy of Xpert MTB/RIF Ultra for the Diagnosis of Pleural TB in a Multicenter Cohort Study. Chest. 2020; 157: 268-275.
- Esmail A, Tomasicchio M, Meldau R, Makambwa E, Dheda K. Comparison of Xpert MTB/RIF (G4) and Xpert ultra, including trace readouts, for the diagnosis of pulmonary tuberculosis in a TB and HIV endemic setting. Int J Infect Dis. 2020; 95: 246 52.
- Singh BK, Jorwal P, Soneja M, Sharma R, Wig N. Trace result in Xpert MTB/ RIF ultra: A diagnostic dilemma and interpretation?. Acta Scientific Medical Sciences. 2021; 5: 58-9.
- Abebe G, Aragaw D, Tadesse M. Fluorescence microscopy for the diagnosis of smear-negative pulmonary tuberculosis in Ethiopia. Afr J Lab Med. 2020; 9: a810.
- Ramachandra V, Brammacharry U, Muralidhar A, Muthukumar A, Mani R, Muthaiah M, et al. Assess the Diagnostic Accuracy of GeneXpert to Detect Mycobacterium tuberculosis and Rifampicin-Resistant Tuberculosis among Presumptive Tuberculosis and Presumptive Drug-Resistant Tuberculosis Patients. Microbiol Res. 2024; 15: 91–108.
- Chien J-Y, Lin C-K, Yu C-J, Hsueh P-R. Usefulness of Xpert MTB/RIF Ultra to Rapidly Diagnose Sputum Smear-Negative Pulmonary Tuberculosis Using Bronchial Washing Fluid. Front Microbiol. 2020; 11: 588963.
- Slail MJ, Booq RY, Al-Ahmad IH, Alharbi AA, Alharbi SF, Alotaibi MZ, et al. Evaluation of Xpert MTB/RIF Ultra for the Diagnosis of Extrapulmonary Tuberculosis: A Retrospective Analysis in Saudi Arabia. J Epidemiol Glob Health. 2023; 13: 782-793.
- Xie YL, Eichberg C, Hapeela N, Nakabugo E, Anyango I, Arora K, et al. Xpert MTB/RIF Ultra versus mycobacterial growth indicator tube liquid culture for detection of Mycobacterium tuberculosis in symptomatic adults: a diagnostic accuracy study. Lancet Microbe. 2024; 5: e520-e528.
- Cao XJ, Li YP, Wang JY, Zhou J, Guo XG. MPT64 assays for the rapid detection of Mycobacterium tuberculosis. BMC Infect Dis. 2021; 21: 336.
- MedCalc: MedCalc’s Relative risk calculator. MedCalc Software Ltd. 2024.
- Piersimoni C, Gherardi G, Gracciotti N, Pocognoli A. Comparative evaluation of Xpert MTB/RIF and the new Xpert MTB/RIF ultra with respiratory and extra-pulmonary specimens for tuberculosis case detection in a low incidence setting. J Clin Tuberc Other Mycobact Dis. 2019; 15: 100094.
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018; 18: 76–84.
- World Health Organization. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Geneva: World Health Organization. 2017.
- Berhanu RH, David A, da Silva P, Shearer K, Sanne I, Stevens W, et al. Performance of Xpert MTB/RIF, Xpert Ultra, and Abbott RealTime MTB for diagnosis of pulmonary tuberculosis in a high-HIV-Burden Setting. J Clin Microbiol. 2018; 56: e00560-18.
- Mishra H, Reeve BW, Palmer Z, Caldwell Dolby T, Naidoo CC, Jackson JG, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respiratory Medicine. 2020; 8: P368-382.