
Review Article
J Bacteriol Mycol. 2015;2(1): 1013.
Probiotics: Insights on Probiotic Effects and Next Generation Therapy to Combat Inflammatory Bowel Diseases
Khan MA and Morshed MN*
Department of Science and Humanities, Military Institute of Science and Technology, Bangladesh
*Corresponding author: Morshed MN, Assistant Professor / Instructor CI ‘B’, Department of Science and Humanities, Military Institute of Science and Technology (MIST), Mirpur Cantonment, Dhaka-1216, Bangladesh
Received: March 21, 2015; Accepted: July 03, 2015; Published: July 06, 2015
Abstract
Probiotics are live organisms that exert a health benefit on the host, have been used for the management of a range of gastrointestinal disorders, though there have little evidence. Interest in intestinal microflora is increased because of its effect on various gastrointestinal disorders. Inflammatory Bowel Diseases (IBD) is a complex, multifactorial, and the most frequent gastrointestinal disorders all over the world. In more recent time, Probiotics have been explored as a possible treatment for IBD and other gastrointestinal diseases. The mode of action of probiotics has not been fully elucidated. The acceptance of probiotics has been better gradually due to the development of modern molecular methods and well-controlled experimental trials of the results of probiotics on IBD to scrutinize and recognize multifarious bacteria within human intestines. The Probiotic strains, molecular mechanism of probiotic action on human intestine, Probiotic action on IBD disease etiology are reviewed. The effect of probiotics on different gastrointestinal disorders and the consequences from experimental trials of probiotics for the treatment of such disorders are also summarized. These areas of research have great potential and deserve more experimental study at all levels.
Keywords: Probiotics; Gastrointestinal disorders; Inflammatory bowel diseases; Clinical trials
Introduction
In the late 19th century, microbiologists identified the differences of microbiota in the Gastrointestinal Tracts (GI) between healthy and diseased individuals. These type of beneficial microbiota found in the GI tract were named probiotics. Probiotics are living microorganisms that beneficially influence the health of humans when ingested in adequate numbers [1]. Probiotic literally means “for life.” The Nobel laureate Metchnikoff is credited with first recognizing the health benefits of probiotics in 1907 suggesting that the consumption of living lactic acid bacteria in fermented foods may promote health and longevity by favorably modulating the gastrointestinal microflora [2]. Over the past century an expanding body of basic and clinical research supports the health benefits of probiotic consumption in a host of disorders including irritable bowel syndrome, inflammatory bowel disease, diarrhea, food allergies, lactose intolerance, urogenital infections, and atopic eczema [3].
The actual introduction of the concept belongs to Lilly and Stillwell in 1965, after which probiotics are characterized as “microorganisms that promote growth of other microorganisms [4]. In 1974, Parker talks about a food supplement for livestock and improve name of probiotics as “organisms and substances that helps the microbial ecosystem” [5]. Their importance was highlighted by Fuller in 1989 who described probiotics as live microorganisms with beneficial effects on host body, improving intestinal microbial balance [6]. Today the universal meaning of the term “probiotic” was established by the World Health Organization and the Food and Agriculture Organization of the United States. These two organizations defined probiotics as “live microorganisms which when administered in adequate amounts, have a beneficial effect on health of the host organism”
A number of studies have found probiotic consumption to be useful in the treatment of many types of diarrhea, including antibiotic-associated diarrhea in adults, travelers’ diarrhea, and diarrheal diseases in young children caused by rotaviruses. The most commonly studied probiotic species in these studies have been found to be Lactobacillus casei sp. (Strain GG), Lactobacillus casei, Bifidobacterium bifidum, Streptococcus thermophilus [7-9].
This literature review addresses three key issues: 1. Probiotics strains, 2. Mechanism of actions of probiotic activity, 3. Mechanism of probiotic activity on Inflammatory Bowel Diseases.
Probiotics
Probiotics are healthy and beneficial microbial food ingredients or dietary supplements that have a useful effect on the host. The effect is influenced by increasing the metabolic activity of the Gastrointestinal (GI) tract flora. Parker defined probiotics: “Organisms and substances which throw into intestinal microbial balance”. Afterward, this was modified to read: “A live microbial feed add-on which beneficially affects the host by developing its intestinal microbial balance.”
Microorganisms used in probiotics
Bifidobacterium and Lactobacillus are the main genera of probiotic microorganisms. Some other bacteria and yeasts (Saccharomyces boulardii) have been used also. Bifidobacterium species and Lactobacillus casei and Lactobacillus acidophilus are used extensively. Enterococci are used rarely as probiotics. Enterococcus faecium (St. SF68) is the best studied enterococci act as probiotics. Sometimes it is considered as alternative of antibiotics for diarrhea treatment.
Lactobacillus acidophilus: Lactobacillus acidophilus is the most widespread probiotics on the present market. It is regularly used in yogurt culture. In accordance with the National Institute of Health (NIH), the most trustworthy use of Lactobacillus acidophilus is in the cure of bacterial vaginosis.
Lactobacillus rhamnosus: Lactobacillus rhamnosus is more expensive than Lactobacillus acidophilus and shows relatively similar effects on human health. It has not been subjected to similar amount of study. The Indian Journal of Medical Microbiology comments that it has established useful affects on intestinal immunity.
Bacillus coagulans: Bacillus coagulans is comparatively rare on the market. It hasn’t been used in commercial foods unlike Bifidobacteriam and Lactobacillus species.
Bifidobacterium animalis: Bifidobacterium animalis can improve digestive activity. It is regularly used for chronic constipation or irritable bowel syndrome. It also used in Danon (the yogurt manufacturer) under the name “Bifidusregularis”.
: are generally considered a toxic species of bacteria. Some strains are not only nonpathogenic but also have therapeutic value. can treat and prevent ulcerative colitis.
Lactococcus lactis: Lactococcus lactis has limited medical value but it has wide commercial value. It also used in buttermilk and cheese production.
i Lactobacillus reuteri is found in the most animal’s colon which is found in human breast milk. It is called the universal probiotic. It can fight with pathogenic bacteria [10-15] (Table 1).
Lactobacillus Sp.
Bifidobacterium Sp.
Enterococcus Sp.
Streptococcus Sp.
Bacillus Sp.
Pediococcus Sp.
Lactobacillus acidophilus,
Lactobacillus delbrueckii,
Lactobacillus casei,
Lactobacillus cellobiosus,
Lactobacillus fermentum,
Lactobacillus curvatus,
Lactobacillus lactis,
Lactobacillus plantarum,
Lactobacillus reuteri.
Bifidobacterium adolescentis,
Bifidobacterium bifidum,
Bifidobacterium infantis,
Bifidobacterium thermophilum,
Bifidobacterium animalis,
Bifidobacterium longum.
Enterococcus faecium,
Enterococcus faecalis.
Streptococcus salivarius,
Streptococcus diacetylactis,
Streptococcus cremoris,
Streptococcus intermedius.
Bacillus cereus vartoyoi,
Bacillus subtilis,
Bacillus licheniformis,
Bacillus coagulans,
Bacillus polyferminticus,
Bacillus laterosporus,
Bacillus pumilus,
Bacillus polymyxa,
Bacillus clausii
Pediococcus cerevisiae,
Pediococcus acidilactici.
Table 1: Table of the most commonly used species of Probiotics [10].
Sources of probiotics
1. Yogurt
Yogurt contains the culture of live bacteria and has beneficial effects on human health.
2. Cheese
Aged cheeses like blue and cheddar cheese are the best sources of probiotics in contrast processed cheese do not contain probiotics.
3. Kefir
Kefir is a good quality source of probiotics. It is a yogurt-like drink. It is not readily available to the general people.
4. Sauerkraut
Sauerkraut is a fermenting cabbage alike German style. It, which is homemade, will have probiotics.
Effects of Probiotics on Intestinal Infections
Probiotics can enhance both the specific and nonspecific immune response, possibly by activating macrophages, increasing natural killer cell activity, increasing levels of cytokines, and increasing levels of immunoglobulins. In spite of limited testing in humans, these results may be particularly important to the elderly, who could benefit from an enhanced immune response.
Diarrhea
In preliminary research, some probiotics have been revealed that they can treat a variety of gastroenteritis. They might decrease both the frequency of stools and the duration of illness [16]. After antibiotic therapy an imbalance is caused in the colonic microbiota. This situation is called Antibiotic-Associated Diarrhea (AAD). Alteration of microbiota changes carbohydrate metabolism with reduced short-chain fatty acid absorption. As a result an osmotic diarrhea is occurred. Another result of antibiotic therapy leading to diarrhea is overgrowth of pathogenic organisms such as Clostridium difficile. Probiotics might reduce the occurrence and severity of AAD as found in a number of meta-analysis. [17-22] For instance Lactobacillus rhamnosus may decrease the risk of AAD, boost up the immune response after vaccination and enhance stool regularity during antibiotic therapy [23]. Probiotic efficacy in AAD depends on the probiotic strain (s) and its dosage [24]. Up to a 50% decline of AAD incidence has been found in primary studies [25]. No side effects have been found in any of these studies. On the other hand, additional documentation of these result through randomized, double blind, controlled trials are necessary to validate precise effects and achieve regulatory authorization, which does not exist right now.
Lactose intolerance
Lactic acid bacteria can convert lactose into lactic acid. Taking certain strains of lactic acid bacteria may assist lactose intolerant persons tolerate additional lactose [26].
Colon cancer
Heterocyclic amines are carcinogenic substances that are produced in cooked meat. Some strains of Lactobacillus delbrueckii sub sp. Bulgaricus (LAB) have verified anti-mutagenic property thought to be because of their capability to bind with heterocyclic amines [27]. There have evidence that some LAB may act against colon cancer in rodents although human data are indecisive [28]. Some clinical trials found that the strains may show anti-carcinogenic effects by reducing the activity of β-glucuronidase [29].
Inflammation
Some strains of Lactobacillus delbrueckii sub sp. Bulgaricus may adjust hypersensitivity and inflammatory responses due to the directive of cytokine function [23]. Clinical studies recommended that they can prevent milk allergies [28] as well as reoccurrences of Inflammatory Bowel Diseases (IBD) in adults [23]. They are ineffective to treat eczema, a skin inflammation [29]. The mechanism of probiotic action with the immune system is unclear till now but a potential mechanism of T lymphocytes response to pro-inflammatory stimuli under research concerns [30].
Irritable bowel syndrome (IBS) and colitis
In a study, a strain of Bifidobacterium infantis can improve some symptoms of IBS in women [31]. Another study found that a strain of Lactobacillus plantarum is also effective in minimizing IBS symptoms [32]. Bifidobacterium animalis can help to stabilize stool frequency with constipation predominant IBS [33]. For the remedy of ulcerative colitis, clinical studies found equivalence of mesalazine (5-ASAs) and mutaflor [34].
Mechanism of Probiotics Activity
In recent years, several studies of probiotic activity give evidence that probiotics respond to experimental and human IBS by their antibacterial activity regarding colonization of the epithelial layer as well as on epithelial cell function includes epithelial cytokine secretion and epithelial cell barrier function. However, there has promising evidence that probiotics can induce regularity T cells which reduce inflammation [33].
Immunomodulation
Immune modulation, the intestinal lymphoid tissue is the largest in size compared with other areas of the body. It is well known that bacteria are critical for the development and functioning of the immune system at this level, being actually the defense mechanism against infection by pathogens [34,35]. Intestinal lymphoid tissue makes contact with the food components, the antigens and with the beneficial or pathogenic bacteria. Antigens, substances that can trigger an immune response, enter the body through the intestinal mucosa that is essential in controlling immunity to invasion of pathogenic bacteria. The adaptability to various antigens is extremely important if we consider that the composition of intestinal mass change very frequently. Most of the antigen is released from first contact with the intestinal mucosa [36]. After crossing the epithelial barrier by transcytosis, they are restructured by a lysosomal degradation processes. A further screening is in the presence of M cells (cells of follicular epithelium associated with lymphoid tissue) followed by the T cells (lymphocyte cells belonging to the group of white blood cells) which are then differentiated as cells that mediate an immune response and promotes cell differentiation and secreting IgA (immunoglobulin A) [37]. IgA is an antibody that plays a crucial role in mucosal immunity. In Figure 1, we are presenting the hypothetical effect in modulating and immune response. Through TLR receptors (Toll Like Receptors), Dendritic Cells (DC) and T cells, probiotics, leads to reduced secretion of TH1 (lymphocyte involved in an enhanced immune response), IL12 (interleukin which is naturally produced by dendritic cells), TNFa (inflammatory cytokine) and IFN-γ (cytokine that is critical for innate and adaptive immunity) which are responsible for the onset of inflammatory processes in the intestines.
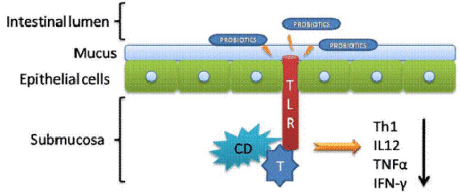
Figure 1: The effect of probiotic bacteria on the immune system [41].
The mechanisms by which epithelial cells are making the difference between probiotic and pathogenic microorganisms appear to be different. Pathogenic bacteria induce a pro-inflammatory response in epithelial cells by activating transcription factor NF-kB. Compared with these bacteria, non-pathogenic species may alleviate to the side of pro-inflammatory response by blocking this factor [38]. It was found that the administration of Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 stimulates activity of cytotoxic lymphocytes. Similar experiments show that administration of these probiotics reduced the activity of these lymphocytes [39,40]. Stimulation of cytotoxic lymphocytes activity is correlated with the [41] secretion of IL-12, another cytokine involved in their activities when Lactobacillus casei Shirota is administered [42]. These studies suggest that probiotics can play an important role in stimulating the activity of cytotoxic lymphocytes having a direct role in preventing the development of malignant tumors’. It also appears that the role of probiotics in phagocytosis and the activity of cytotoxic lymphocytes are vital especially in the elderly, who have a compromised immune system [43-45]. Quality and dose of probiotic preparations influence the IL-8 secretion via the enterocites. IL-8 is associated with the development of intestinal inflammation. Recent data shows that when incubated with Lactobacillus rhamnosus Strain GG the CaCO-2 cells (intestinal epithelial cell) reduces the amount ofIL-8 produced [46]. In many cases was shown that enterocytes produce IL-8 and other cytokines in the presence of probiotics such as IL-6 [47]. IL-6 stimulation was achieved by administering Lactobacillus casei CRL431 and Lactobacillus helveticus R389 [48]. In conclusion studies to date show that each probiotic is characterized in regards to its influence on the immune system. In other words, bacteria have immune modulatory qualities characteristic of each one. Next objective would be to determine the exact components of each probiotic strain that are or may be directly involved in triggering an immune response. Probiotics can influence the immune system by different metabolites, the cell wall components and DNA.
Inhibition of pathogenic bacteria
The gastrointestinal environment contains a wide range of contents ranging from bacteriocins, in situ in the intestine can be progressed by increasing the capability of probiotic bacteria to adhere to the intestinal mucosa. Bovine colostrum contains substances that can triple the capacity of Lactobacillus casei species to adhere to intestinal cell line Caco-2. However, in situ production of microbial substances adversely affect intestinal microflora beneficial to the host organism [49]. Ruminal bacteria can also produce such bacteriocins which by their presence are able to modify the harmful pathogenic bacteria. The mammalian organism fights against these pathogenic bacteria through various ways : blocking pathogenic bacteria effects by inducing bactericidal substances and fighting with pathogens and toxins for devotion to the intestinal epithelium; regulation of the immune responses by improving the innate immunity and amending pathogen-induced inflammation through toll-like receptor regulated signaling pathways; adjust intestinal epithelial homeostasis by promoting intestinal epithelial cell survival, stimulating protective responses and enhancing barrier function (Figure 2) [41].
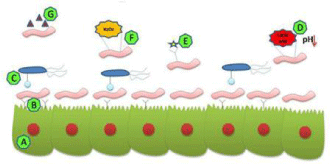
Figure 2: Graphical representation of the mode of action of probiotics in the
intestines [41].
The approach is based on the capability of probiotics (A) to tie pathogens (B) in intestinal epithelial tissue (C). Probiotic action consists in production of lactic acid (D) which reduces the pH, interacts with the toxins induced by pathogens (E), with the production of hydrogen peroxide (F) and synthesis bacteriocins (G). Some studies even recommend using ruminal bacteriocins as an alternative to antibiotics in cattle [50]. In vitro studies have shown that strains of lactic acid bacteria are effective in removing or stopping the activity of pathogenic bacteria. Studies in vitro with human cell lines have helped to investigate how probiotics adhere to the intestinal epithelium. These cell lines have different phenotypic characteristics and they have been widely used especially in humans [51]. Their use has its explanation in the fact that mimics the intestinal barrier that pathogenic microorganisms must pass in order to infect and then systemic circulation to reach various parts of the body [52]. Production of assured metabolites such as lactic acid inferiors the pH with a crucial role in inhibiting the growth of pathogenic bacteria. But there are also cases where pathogen inhibition (Shigella) is due not only to pH but also to some antibacterial substances secreted by lactic acid bacteria [53]. Hydrogen peroxide secretion is also a significant factor and was identified as having inhibitory consequence on growth and development of 0157: H7 [54]. Supernatants derived from Lactobacillus rhamnosus Lcr35 cultures had an inhibitor effect on nine types of pathogenic bacteria: Clostridium difficile, (EPEC), (ETEC), Enterobacter cloacae, Klebsiella pneumoniae, Salmonella typhimurium, Shigella flexneri, Pseudomonas aeruginosa, and Enterococcus faecalis [55]. Competitive exclusion of pathogens can be used efficiently to farm animals after treatment with antibiotics to prevent infection with Salmonella during especially because the host microflora is in recovery. This concept involves administration of non-pathogenic bacteria cultures (one or more strains) in order to reduce colonization or presence of pathogenic bacteria in the intestine [56].There is therefore sufficient evidence to demonstrate the use of probiotics in maintaining control of Helicobacter pylori colonization of gastric mucosa. Clinical studies and experimental animal models have shown that Lactobacillus acidophillus can affect growth and development of this pathogen both in vitro and in vivo [57]. However to date there is insufficient data to suggest the use of probiotics in the absence of antibiotics to prevent infection with H. pylori. Administration of probiotics (Lactobacillus rhamnosus HN001) in animals, under experimental conditions, resulted in an improved immune response following Salmonella enterica infestation [45]. It is also interesting that the animals who were artificially infected with Salmonella and which received probiotics have synthesized high levels of serum antibodies leading to increased survival to infection but also to a decrease in the presence of these pathogens in liver and spleen. The same effects have been identified when Lactobacillus salivarius CTC2197 is administered to Leghorn birds [58]. Given this we can say that probiotics help the digestive tract by competing with pathogenic bacteria for the adhesion sites. If they manage to cross the epithelial barrier will trigger an immune response and antibody production, a process that is mediated by the probiotic and will lead to pathogenic bacteria eradication. Prevention of infections by Listeria monocytogenes is a topic of great interest particularly for poultry. Bacteriocins produced by Enterococcus faecium SH528, SH632, Pediococcus pentosaceus, Enterococcus faecium SH740 were proven to be effective in combating Listeria monocytogenes [59]. Studies on rats artificially infected with Listeria show that administration of Lactobacillus casei lead to reduced presence of pathogens in particular the liver [60]. Efficacy of probiotics was also proven in urogenital infections and was tested by studies performed on healthy patients or female patients who were diagnosed with uro-vaginal infections. Results from these studies suggest beneficial effects of the use of probiotics in preventing urinary tract infections [61]. However clinical research should be expanded, especially for commercial products to increase their effectiveness and in particular to accurately identify their spectrum of action. So far have been suggested several mechanisms by which probiotics are involved in preventing the harmful effect of intestinal pathogens such as competition for nutrients, inhibition of interaction between pathogens and intestinal mucosa, production of antimicrobial substances and stimulation of mucosal immunity [56]. However, there are many aspects of interaction between pathogens and probiotics which are of great interest for many researchers in the field aiming to understand the anti-pathogenic mechanism of probiotics.
Effects of Probiotics on Different GI Disorders
Gastrointestinal (GI) disorders are including functional bowel diseases such as Irritable Bowel Syndrome (IBS), colon cancer, constipation, lactose intolerance, and inflammatory bowel diseases such as Crohn’s Disease (CD) and Ulcerative Colitis (UC). Symptoms of GI disorders often include cramping, abdominal pain, inflammation of the lining of the large and/or small intestine, chronic diarrhea, rectal bleeding and weight loss. Near the beginning studies had shown that some intestinal bacteria are associated with the production of carcinogens, pro-carcinogens or co-carcinogens. As a result of toxin production, colon cancer is initiated [62]. Another earlier study has found that 20% of colon cancer was induced chemically by germ free animals. In contrast 93% of colon tumors were induced by their counterparts with a normal flora [63]. Reddy et al. were used azoxymethane to induce aberrant crypt loci in rats. They found that Bifidobacteria could inhibit colon carcinogenesis in the colon. The authors also suggested that this type of inhibition of crypt multiplicity and crypt foci was featured in the pH lowering consequence of Bifidobacteria in colon. It was also inhibited the production of clostridia and E. coli. Bacterial enzymes, betaglucuronidase can convert pro-carcinogens to proximate carcinogens [64]. Probiotics for example Bifidobacterium produce several metabolites that could influence the function of P450s and afterward have an effect on the conversion of azoxymethane (proximate to ultimate carcinogen) [65]. This result gives indication that probiotics could repress colon cancer. Further investigations have also found that cultured milk have desmutagenicity which increase the number of viable cells. Viable cells play an essential role in mutagenicity [66]. A study by Thyagaraja and Hosono found that probiotics segregated from a traditional cereal pulse product of India named ‘idly’ could show desmutagenicity on various heterocyclic amines, spice mutagens and aflatoxins [67]. Later studies on the desmutagenicity properties of probiotics found that the desmutagenic particles may be located in the cellular envelope of the bacterial cell wall [63]. Bifidobacterium infantis can also inhibit tumorogenic activity in the cell wall preparation of mouse peritoneal cells in vitro [68]. Another study on heat killed Lactobacillus casei (LC9018) was found that it could induce immunity against tumor introduction in a controlled, randomized and comparative study on 223 patients with cervical cancer (stage III). The antitumor activity was found because of its macrophage activation by LC9018 [69]. Mutagens were bound to the cell wall of probiotic organisms. This result has been supported by earlier studies that have found attaching characteristics of probiotics on mutagens [70] and the binding study of heterocyclic amines by intestinal probiotics [71].
Lactobacilli acidophilus are natural probiotic microorganisms that are found in the human digestive tract. They work in several ways to promote human health. Some of them include inhibiting the growth of harmful bacteria, increasing the self-protective activity of gastrointestinal tract, improving immunity and helping in vitamin K production. Recent study has found the evidence of the efficiency of probiotics in the treatment of constipation, diarrhea and bloating. Another study found that Lactobacillus casei Shirota (LcS) have beneficial effect on Chronic Constipation (CC). They recommended probiotic foods as an adjunctive therapy of CC. A pilot study also finds that mixtures of Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus in combination have positive effects on constipation. More studies will be upcoming in near future. After that Probiotic therapy will be very popular substitute of health care system [72-74].
Lactose intolerance is occurred by absent or limited production of lactase. Small intestine can’t digest lactose or milk sugar due to lack of lactase. It goes into lower GI tract to break down and utilize its energy as an alternative. Thus gas is produced and leading to lactose intolerance. Some scientific studies recommend consuming probiotics, particularly Lactobacillus acidophilus, can assist to reduce the symptoms of this disorder and digest lactose, milk and additional dairy products easily. For example, Montes R et al. has been concluded that probiotics Lactobacillus supplements can reduce lactose intolerance in milk consumed children than those who consumed milk with no probiotic supplements. Few articles also claim that probiotics couldn’t alleviate the warning sign of lactose intolerance but they recommend the use of Lactobacillus is quite harmless [75,76].
Initiation and perpetuation of intestinal inflammation on Inflammatory bowel diseases (IBD) and probiotic effects
The Gastrointestinal Tract hosts a wide range of bacterial species such as Firmicute (~ 64 %), Bacteroidetes (~ 23%), Proteobacteria, and Actinobacteria. Evidence suggests that the intestinal microorganism play a significant role in initiating, maintaining, and shaping the phenotype of IBD [77,78]. CD and UC preferably occur in colon and/ or ileum due to the dysbiosis of intestinal microbiota. A possible direct relationship is associated between bacterial numbers and disease in this regards although the actual composition of the microbiota remains unclear [79]. Later another study found that Bacteroidetes and Lachnospiraceae (a Firmicute) were greatly reduced while Proteobacteria and Actinobacteria were substantially more abundant in IBD patients than former. These findings proved dysbiosis as a crucial characteristic of IBD [80]. Sartor and Sarkis concluded that commensal bacteria ( Clostridium difficile, Bacteroides fragilis) and pathogens (Mycobacterium avium subspecies paratuberculosis, enterotoxigenic Bacteroides fragilis) induce IBD as consequence of intestinal inflammation besides host genetic defects in commensal bacteria and defective host immunoregulation notified as important concern for IBD [81].
The intestinal tracts of mammals contain a large diversity of pathogenic and nonpathogenic microbes. Much attention was given in the past to identify the mechanism of secreting harmful effects by pathogenic bacteria. On the other hand, more modern research has found that probiotics played crucial role in human health care. Probiotics, synbiotics and prebiotics are going into the conventional of health care system. The acceptance of probiotics has been increased day by day due to the advancement of modern molecular methods and well-controlled clinical trials of the results of probiotics on IBD to analyze and identify multifarious bacteria within human intestines. In recent years, some review literatures have been published describing the efficacy of synbiotics, prebiotics and probiotics on IBD [82,83] .
Mechanisms of action of probiotic bacteria in inflammatory bowel disease
It is crystal clear that considerable differences exist between probiotic bacterial species and strains. Earlier research on probiotics has paid attention on their safety and aptitude to live on gastrointestinal transit relative to their application in the agriculture and food industry. On the other hand, throughout the past several years, important advances have been made in understanding the mode of action of individual strains as they relate to the pathophysiology of IBD. This will subsequently permit the development of definitive criteria for choice of probiotic strains for exact clinical indications. It will also allow for the determination of best possible doses, timing of administration, and probable synergy between bacterial species.
Presently, useful effects of probiotics may be generally classified into two major categories: (1) those effects arising as a result of activity in the large intestine linked to colonization and inhibition of pathogen growth and (2) those effects associated to enrichment of the host immune response and barrier function during interactions with epithelial and immune cells within both the small and large intestine. It is becoming clear that bacterial strains can modulate the function of the immune system at both a systemic and a mucosal level in the intestine. Immune cells are frequently sampling and responding to probiotics [84]. Additionally, various bacterial strains can signal through pattern-recognition receptors, in that manner modulating different intracellular signaling pathways [85,86]. Active constituents of bacteria that influence the mucosal immune system include enzymes [87]; secreted protein factors; peptides such as Lipopolysaccharide (LPS) and N-Formylmethionine- Leucine-Phenylalanine (fMLP) [86,88]; and peptidoglycan cell wall constituents counting the Muramyl Dipeptide (MDP), gamma- D-glutamyl-meso-diaminopimelic acid (iE-DAP) and bacterial Deoxyribonucleic Acid (DNA). There are currently five, probable interconnected, probiotic modes of action relative to therapy for IBD: (1) receptor competition, whereby probiotics contend with microbial pathogens for a partial number of receptors at hand on the surface epithelium; (2) immunomodulation and/or activation of immune function of gut-associated lymphoid and epithelial cells; (3) pathogen growth suppression by probiotics through liberate of antimicrobial factors; (4) probiotic induced development of mucosal barrier function; and (5) initiation of T-cell apoptosis in the lamina propria (Figure 3).
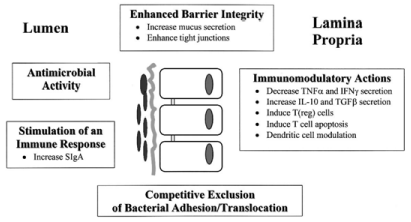
Figure 3: Mechanisms of action of probiotics in therapy for inflammatory
bowel disease [89].
It still remains to be recognized which latent mechanism (s) relate to the treatment of IBD and how the pathophysiology of clinical disease is changed through utilization of probiotics. Nevertheless, it is clear from these experimental models that probiotics vary significantly in their modes of action, and that a distinct mechanism of action is unlikely to be accountable for their clinical effects. In addition to precise interactions between probiotics and host immune cells, microbe-microbe interactions also subsist. This could clarify some of the varying results found within the different clinical trials. One of the most commonly accepted theories of IBD is that acute and chronic inflammation results from an interaction among susceptibility genes, luminal microflora, and a dysregulated immune system. It is to be expected that upcoming research will result in the identification of clinical phenotypes in patients with IBD that act in response to probiotic therapy and that definite strain will be characterized in order for a targeted therapeutic approach to occur [89].
Using probiotics to combat IBD: scientific rationale
Several clinical studies conducted in more recent time applying different probiotic combination in IBD patients and found promising results [90-95].
Gosselink et al. conducted a study on 117 patients randomly with UC to Lactobacillus rhamnosus strain GG daily (39 patients) or without treatment (78 patients). After that 1st periods of pouchitis were found significantly fewer frequently among patients who accepted the probiotic [96]. Another study demonstrated by Kruis et al. that E. coli Strain Nissle 1917 was comparable to low-dose mesalamine in declining relapse of quiescent UC [97]. Probiotics can help to prevent the regeneration of ulcerative colitis. A study by Mario Guslandi et al. have been found that Saccharomyces boulardii, a probiotic yeast cured 17 of 24 patients in 2003. Other studies by Hai-Hong Cul et al. VT Do et al. found BIFICO (mixture of probiotics) and 2 probiotic strains such as Bifidobacterium and Lactobacillus rhamnosus Strain GG can reduce occurrence and improve remission time of ulcerative colitis [98-100]. A randomized, placebo-controlled clinical trial of the probiotic Lactobacillus Strain GG was unable to conclude that this probiotic strain would extend remission time in patients with CD which was already in remission on a standard therapy [101]. A literature review of five studies in adults and one more in children conclude that presently the data do not support the utilization of probiotics in adult or children with CD [102].
Conclusion
In this review, the effects and application of probiotic microorganisms in IBD was reviewed. Human has entered the era when the use of antibiotics or other pharmaceutical products is increasingly annoying. Antibiotics are actually banned by educated human for their extreme side effects as a nutritional supplement. Probiotics will be the best therapy in future for treating many gastrointestinal diseases. However, more evidence-based research is required for an in-depth evaluation of probiotics in medical science before they can be safely used.
References
- FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. 2001; 1-4.
- Paraschiv D,Vasile A, Constantin M, Ciobanu A, Bahrim G. Study Of Physiological Properties Of Some Probiotics In Multiple Cultures With Mesophilic Lactic Acid Bacteria By Flora Danica Ch. Hansen Commercial Starter. The Annals Of The University Dunarea De Jos Of Galati Fascicle Vi – Food Technology. 2011; 35: 56-65.
- Metchnikoff E. The Prolongation of Life. Optimistic Studies. London. 1907; 161-183.
- Lilly DM, Stillwell RH. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science. 1965; 147: 747-748.
- Parker R. Probiotics, the other half of the antibiotic story. Anim Nutr Health. 1974; 28: 240-255.
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989; 66: 365-378.
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp. Strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991; 88: 90- 97.
- Oksanen PJ, Salminen S, Saxelin M, Hamalainen P, Ihantola-Vormisto A, Muurasniemi-Isoviita L, et al. Prevention of traveler's diarrhea by Lactobacillus GG. Ann Med. 1990; 22: 53-56.
- Siitonen S, Vapaatalo H, Salminen S, Gordin A, Saxelin M, Wikberg R, et al. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann. Med. 1990; 22: 57-59.
- Adam JA, Odhav B, Naidu KSB. Probiotics: Recent Understandings and Biomedical Applications. Curr Trends Biotechnol Pharm. 2012; 6: 1-14
- Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr. 2000; 54: 263-267.
- Ohland CL, Macnaughton WK . Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010; 298: G807-G819.
- Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005; 128: 541-551.
- Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterol. 2004; 126: 520-528.
- Zoetendal EG, Collier CT, Koike S, Mackie RI, Gaskins HR. Molecular ecological analysis of the gastrointestinal microbiota: a review. J Nutr. 2004; 134: 465-472.
- King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003; 52: 1-16.
- Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010; 11: CD003048.
- D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002; 324: 1361.
- Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002; 16: 1461-1467.
- Mcfarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006; 101: 812-822.
- Szajewska H, Mrukowicz J. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2005; 22: 365-372.
- Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006; 149: 367-372.
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006; 6: 374-382.
- Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol. 2008; 42: S58-S63.
- Surawicz CM. Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2008; 42: S64-S70.
- Sanders ME. Considerations for use of Probiotic bacteria to modulate human health. J Nutr. 2000; 130: 384S-390S.
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr. 2003; 36: 223-227.
- Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for treating eczema. Boyle, Robert John. edn. Cochrane Database Syst Rev. 2008.
- Braat H, Van Den Brande J, Van Tol E, Hommes D, Peppelenbosch M, Van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004; 80: 1618-1625.
- Hitti Miranda. Probiotics May Help Stressed Gut.
- Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001; 13: 1143-1147.
- Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007; 26: 475-486.
- Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014; 17: 466-470.
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999; 69: 1046S-1051S.
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998; 62: 1157-1170.
- Sanderson IR, Walker WA. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update). Gastroenterol. 2003; 104: 622-639.
- Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998; 18: 1-30.
- Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000; 289: 1560-1563.
- Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001; 21: 264-271.
- Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001; 20: 149-156.
- Corcionivoschi N, Drinceanu D. Probioticele-la timpul prezent. 2009.
- Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin- 12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006; 146: 109-115.
- Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr. 2000; 54: 263-267.
- Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001, 74: 833-839.
- Gill HS, Shu Q, Lin H, Rutherfurd KJ, Cross ML. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med Microbiol Immunol. 2001; 190: 97-104.
- Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005; 135: 1752-1756.
- Ruiz PA, Hoffmann M, Szcesny S, Blaut M, Haller D. Innate mechanisms for Bifidobacterium lactis to activate transient proinflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology. 2005; 115: 441-450.
- Vinderola G, Matar C, Perdigon G. Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: involvement of toll-like receptors. Clin Diagn Lab Immunol. 2005; 12: 1075-1084.
- Sanders ME. Effect of consumption of lactic cultures on human health. Adv Food Nutr Res. 1993, 37: 67-130.
- Russell JB, Mantovani HC. The bacteriocins of ruminal bacteria and their potential as an alternative to antibiotics. J Mol Microbiol Biotechnol. 2002; 4: 347-355.
- Louvard D, Kedinger M, Hauri HP. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992; 8: 157-195.
- Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998; 60: 161-177.
- Apella MC, Gonzalez SN, Nader de Macias ME, Romero N, Oliver G. In vitro studies on the growth of Shigella sonnei by Lactobacillus casei and Lact. acidophilus. J Appl Bacteriol. 1992; 73: 480-483.
- Brashears MM, Reilly SS, Gilliland SE. Antagonistic action of cells of Lactobacillus lactis toward O157:H7 on refrigerated raw chicken meat. J Food Prot. 1998; 61: 166-170.
- Forestier C, De Champs C, Vatoux C, Joly B. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol. 2001; 152: 167-173.
- Steer T, Carpenter H, Tuohy K, Gibson GR. Perspectives on the role of the human gut microbiota and its modulation by pro- and prebiotics. Nutr Res Rev. 2000; 13: 229-254.
- Felley C, Michetti P. Probiotics and Helicobacter pylori. Best Pract Res Clin Gastroenterol. 2003; 17: 785-791.
- Pascual M, Hugas M, Badiola JI, Monfort JM, Garriga M. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl Environ Microbiol. 1999; 65: 4981-4986.
- Shin MS, Han SK, Ji AR, Kim, KS, Lee WK. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol. 2008; 105: 2203-2212.
- Sato K. Enhancement of host resistance against Listseria infection by Lactobacillus casei: role of macrophages. Infect Immun. 1984; 44: 445-451.
- Petricevic L. and Witt A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG. 2008; 115: 1369-1374.
- Drasar BS, Hill MJ. Human Intestinal Flora. Academic Press Inc. New York. 1974.
- Singh J, Rivenson A, Tomita M, Shimamura S, Ishibashi N, Reddy BS. Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis. 1997; 18: 833-841.
- Kulkarni N, Reddy BS. Inhibitory effect of Bifidobacterium longum cultures on the azoxymethane-induced aberrant crypt foci formation and fecal bacterial β-glucuronidase. Proceedings of the Society for Experimental Biology and Medicine. 1994; 207: 278-283.
- Campbell TC, Hayes JR. The effect of quantity and quality of dietary protein on drug metabolism. Federation Proceedings. 1976; 35: 2470-2474.
- Usman, Hosono A. Desmutagenicity of milk cultured with Lactobacillus acidophilus strains against mutagenic heated tauco. Food Chemistry and Toxicology. 1998; 36: 805-810.
- Thyagaraja N, Hosono A, Nagappa, Akiyoshi. Antimutagenicity of lactic acid bacteria from “Idly” against food related mutagens. J Food Prot. 1993; 56: 1061-1066.
- Sekine K, Ohta J, Onishi M, Tatsuki T, Shimokawa Y, Toida T, et al. Analysis of antitumor properties of effector cells stimulated with a Cell Wall Preparation (WPG) of Bifidobacterium infantis. Biological and Pharmaceutical Bulletin.1995; 18: 148-153.
- Okawa T, Niibe H, Arai T, Sekiba K, Noda K, Takeuchi S, et al. Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. Cancer. 1993; 72: 1949-1954.
- Zhang XB, Ohta Y. Binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria on mutagens. J Dairy Sci. 1991; 74: 1477-1481.
- Orrhage K, Sillerstrom E, Gustaffson JA, Nord CE, Rafter, J. Binding of mutagenic heterocyclic amines by intestinal and lactic acid bacteria. Mutation Research. 1994; 311: 239–248.
- Quigley EM. What is the evidence for the use of probiotics in functional disorders? Curr Gastroenterol Rep. 2008; 10: 379-384.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003; 17: 655-659.
- Bekkali NL, Bongers ME, Van den Berg MM, Liem O, Benninga MA. The role of a probiotics mixture in the treatment of childhood constipation: a pilot study. Nutr J. 2007; 6: 17.
- Montes RG, Bayless TM, Saavedra JM, Perman JA. Effect of Milks Inoculated with Lactobacillus acidophilus or a Yogurt Starter Culture in Lactose-Maldigesting Children. J Dairy Sci. 1995; 78: 1657-1664.
- Levri KM, Ketvertis K, Deramo M, Merenstein JH, Amico FD. Do probiotics reduce adult lactose intolerance? A systematic review. J Fam Pract. 2005; 54: 613-620.
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008; 134: 577-594.
- Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010; 139: 1816-1819.
- Swidsinski A, Ladhoff A , Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, et al. Mucosal flora in inflammatory bowel disease . Gastroenterology 2002; 122: 44-54.
- Frank DN, St Amand AL, Feldman RA, Boedeker ECF, Harpaz N , Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007; 104: 13780-13785.
- Sartor RB and Mazmanian SK. Intestinal Microbes in Inflammatory Bowel Diseases. Am J Gastroenterol 2012; 1: 15-21.
- Marteau PR. Probiotics in clinical conditions. Clin Rev Allergy Immunol. 2002; 22: 255–273.
- Marteau P, Boutron-Ruault MC. Nutritional advantages of probiotics and prebiotics. Br J Nutr. 2002; 87: S153-S157
- Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003; 35: 555–562.
- Anton P, O’Connell J, O’Connell D, Whitaker L, O'Sullivan GC, Collins JK, et al. Mucosal subepithelial binding sites for the bacterial chemotactic peptide, formyl-methionyl-leucylphenylalanine (FMLP). Gut. 1998; 42: 374-379.
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003; 278: 5509-5512.
- Madsen K, Jijon H, Yeung H. DNA from probiotic bacteria exerts anti-inflammatory actions on intestinal epithelial cells by inhibition of NF-kB. Gastroenterol. 2002; 122: A546.
- Lay-Gaik Ooi, Min-Tze Liong. Mechanisms in controlling cholesterol by probiotics. Int J Mol Sci. 2010; 11: 2499-2522.
- Fedorak RN, Madsen KL. Probiotics and the Management of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2004; 10: 286-299.
- Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta- analysis of randomized controlled trials. Inflamm Bowel Dis. 2014; 20: 21-35.
- Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, et al. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after lleo-caecal resection. Inflamm Bowel Dis. 2007; 13: 135-142.
- Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of post operative recurrencein Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006; 55: 842-847.
- Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption– a randomized double-blind placebo-controlled study inactive Crohn’s disease. Aliment Pharmacol Ther. 2010; 32: 872-883.
- Petersen AM, Mirsepasi H, Halkjær SI, Mortensen EM, Nordgaard-Lassen I, Krogfelt KA. Ciprofloxacin and probiotic Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. J Crohns Colitis. 2014; 8: 1498-1505.
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010; 105: 2218-2227.
- Gosselink MP, Schouten WR, van Lieshout LM, et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004; 47: 876-884.
- Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, et al. Maintaining remission of ulcerative colitis with the probiotic Nissle 1917 is as effective as with standard mesalazine. Gut 2004; 53: 1617-1623.
- Guslandi M, Giollo P, Testoni PA. A Pilot Trial of Saccharomyces boulardii in Ulcerative Colitis. Eur J Gastroenterol Hepatol. 2003; 15: 697-698.
- Do VT, Baird BG, Kockler DR. Probiotics for Maintaining Remission of Ulcerative Colitis in Adults. Ann Pharmacother. 2010; 44: 565-571.
- Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, et al. Effects of Probiotics on Intestinal Mucosa of Patients with Ulcerative Colitis. World J Gastroenterol. 2004; 10: 1521-1525.
- Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005; 11: 833-839.
- Guandalini S. Update on the role of probiotics in the therapy of pediatric inflammatory bowel disease. Expert Rev Clin Immunol. 2010; 6: 47-54.