
Research Article
J Bacteriol Mycol. 2015; 2(2): 1018.
Antibacterial Activities of Palladium (II) Complexes Derived from Chitosan Biopolymer Schiff Base
Vadivel T¹*, Dhamodaran M² and Singaram K¹
¹Research and Development Centre, Bharathiar University, Coimbatore, Tamilndu, India
²Department of Chemistry, Perunthalaivar Kamarajar Institute of (Govt.) Engineering and Technology, India
*Corresponding author: Vadivel T, Research Scholar, Research and Development Centre, Bharathiyar University, Coimbatore, Tamilnadu, India
Received: December 14, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Chitosan is a biopolymer, contains polymeric amine which can be modified chemically by condensation reaction with aldehyde in homogeneous phase. This polymeric primary amine (-NH2) of chitosan is functioning for the chemical modification with 4-hydroxy-3-methoxy benzaldehyde, and 2-hydroxy benzaldehyde to form corresponding schiff base derivatives. This schiff base derivatives are becomes a complexing agent or ligand. The Palladium (II) complex was obtained by complexation of Palladium with schiff base ligands and this product exhibits as an excellent solubility and more biocompatibility. The novel series of schiff base Palladium (II) complexes were characterized by Elemental analysis, spectral analysis to confirm the structure and functional groups of chitosan schiff bases, also their metal complex. The synthesized complex has been subjected to antibacterial study. The synthesized chitosan schiff base metal complexes are showed significant antibacterial result against pathogenic bacteria. These findings are giving suitable support for developing new antibacterial drugs and this has motivated us to expand our research and scope for applications.
Keywords: Chitosan; Schiff base; Palladium (II) complex; Antibacterial study
Introduction
The Chemistry of Schiff base and their metal complexes kindled remarkable attentions to the researchers, due to the important development of new drugs and biological important compounds. In this view of motivation, chitosan biopolymer has been chosen to synthesis a new series of drugs. Chitin and chitosan are the second most abundant natural polymers after cellulose and are nitrogenous polysaccharides [1]. They can be extracted from fungi, insects, lobster, shrimp and krill. Crabs obtained from seafood processing waste are an important commercial source [2], it can also be extracted from the exoskeleton of insects and in the cell walls of fungi [3-6].
Chitosan consists of (1-4) - linked β-D-glucosamine (N-acetyl glucosamine) units [7,8], and possesses important physiological properties such as biocompatible, biodegradable, and non-allergenic and this shows various functional properties, which has been applied (or used) in the fields of pharmaceutical, medicinal, biomedical etc. The amine and hydroxyl group of glucosamine present as the repeating unit of the chitosan well behaves as reactive site for the chemical modification [9], which can improve their complexation property with many transition metal ions to form stable complexes. Chitosan has film forming properties and it forms good film and membrane. This attributed the good antibacterial characteristics while it is used in various applications [10]. The interest in Schiff bases and their complexes containing oxygen and nitrogen donor atoms is caused by their significant antimicrobial activity [11]. Additionally, transition metal-schiff base complexes have been found to exhibit some biological properties like antibacterial and antifungal activities [12,13]. In this paper, we have characterized the Palladium (II) complexes derived from schiff base ligands through which the antibacterial activities of Palladium (II) complexes are carried out.
The important pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes are causes for many diseases and poses significant problems to human health. The effective control of these disease causing bacteria is performed by using the synthesized schiff base Palladium (II) complexes. So, antibacterial activity of these complexes against some kinds of bacteria was estimated. The transition metal ions enhance the biological activity of different schiff ligands, and this increased biological activity attributed by metal ion. In future, this work will be focused on biomedical areas, including drug delivery, biosensors, biodegradable packaging, and wound dressing etc.
Materials and Methods
Chitosan was purchased from Sigma-Aldrich Chemical Co., Bangalore, India with a deacetylation percentage in the range of 85 – 90%. All aldehydes were obtained from Merck, Mumbai, India. Acetic acid and ethanol (AR) were obtained from SD fine chemicals, Mumbai. Palladium Chloride (98% Purity) was obtained from Avra Synthesis, Hyderabad, India.
Instrumentation
The FTIR spectra were measured with FT-IR Shimadzu, Prestige-21 Spectrophotometer. Thermal analysis measurement were made in the 40°C – 600°C range at a heating rate 10°C minimum, using Alumel, Perk alloy, and Iron as the references, on a Pyris-6 analyzer TGA – 4000.
Preparation of chitosan – 4-hydroxy-3-methoxy benzaldehyde (CVSB)
CS-4Hdmb was prepared by the condensation of a suspension of 1 g of chitosan with small amount of acetic acid and 0.82 g of 4-hydroxy-3-methoxy benzaldehyde in 20 ml of ethanol. This mixture was stirred at room temperature for 1 h followed by heating for 12 h under water bath at 60°C. The yellow colour product was filtered off, washed with ethanol and dried in vacuo.
Preparation of chitosan – 2-hydroxy benzaldehyde (CSSB)
About 1 g of chitosan powder was dissolved in 25 ml of ethanol with a small amount of acetic acid, shake well until chitosan emulsion was obtained. By adding slowly to the solution of 0.87g of 2-hydroxy benzaldehyde was also dissolved in 25ml ethanol. The solution mixture was stirred, and heated for 12h under water bath at 60°C. The bright yellow colour product was filtered, washed with ethanol and dried in vacuo.
Preparation of schiff base palladium (II) complexes: [Pd(CVSB)].Cl2 and [Pd(CSSB)].Cl2
To a suspension of schiff base ligands (0.2g) in 10ml of ethanol, and it was added to the solution of PdCl2 (0.15g in ethanol). The mixture was stirred and heated for 12h under water bath at 60°C. The brown colour product was filtered off, washed with excess of ethanol and dried in vacuo.
Procedure for measurement of antibacterial activities of Palladium (II) complexes
The test organisms were sub-cultured using nutrient agar medium. The tubes containing sterilized medium were inoculated with the respective bacterial strain. After incubation at 37°C±1°C for 18 hours, they were stored in a refrigerator. The nutrient agar medium was sterilized by autoclaving at 121°C for 15 min. The petriplates, tubes and flasks plugged with cotton were sterilized in hot-air oven at 160 °C, for an hour. To each sterilized petriplate (20cm diameter), was poured about 125ml of molten nutrient agar medium which was already inoculated with the respective strain of bacteria (5ml of inoculum to 250ml of nutrient agar medium) aseptically. The plates were left at room temperature aseptically to allow the solidification. After solidification, the paper discs containing the derivatives were placed at different areas on the surface of each plate and labelled accordingly.
Each test compound (5mg) was dissolved in dimethyl sulfoxide (5ml Analar grade) to give a concentration of 1000μg/ml. Ciprofloxacin solution was also prepared to give a concentration of 1000μg/ml in sterilized distilled water. The pH of all the test solutions and control was maintained in between 2 to 3 by using conc. HCl. All the compounds were tested at dose levels of 1000μg and DMSO used as a control. The solutions of each test compound, control and reference standard were added separately in the cups and the plates were kept undisturbed for at least 2 hours in a refrigerator to allow diffusion of the solution properly into nutrient agar medium. Petri dishes were subsequently incubated at 37±10C for 24 hours. After incubation, the diameter of zone of inhibition surrounding each of the cups was measured with the help of an antibiotic zone reader.
Results and Discussion
Structural confirmation by infra spectroscopy
The FT-IR spectra of chitosan exhibits strong peak at 3379cm- 1 which can be assigned due to coaxial stretching vibration of O-H superimposed to the N-H stretching band and inter hydrogen bands of the polysaccharide. The C-H axial stretching band arises at 2881cm-1. The bands at 1851cm-1 and 1554cm-1 are due to acetyl unit with C = O stretching and N-H bending vibration respectively. Besides, the band at 1151cm-1 due to C-O stretching (1- 4)-linked-β- D-glucosamine unit [14] (Figure 1).
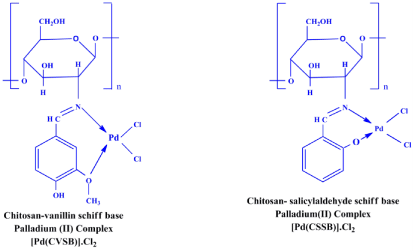
Figure 1: Chemical structure of antibacterial drugs.
The FT-IR spectrum of the Schiff base ligands show a strong band at 1649cm-1, 1629cm-1 which are characteristic stretching frequency of azomethine group (C= –N). These bands are shifted to higher frequency such as 1610cm-1, 1610cm-1 due azomethine group complexed with metal ion. This indicates that the coordination of metal centre with azomethine nitrogen group [15,16]. The band arrived at 1076cm-1 which confirms that the phenolic group of chitosan-salicylaldehyde schiff base (CVSB) coordinated with metal ion for complex formation. The bands arrived at 1070cm-1 in the IR spectrum of both Schiff bases, which are characterized due to C-O-C symmetric stretching of methoxy (R-O-CH3). Further, the band at 2920cm-1 in the IR spectrum of complex has been assumed as 4-OH is not affected and also it is confirmed that methoxy group of chitosanvanillin schiff base made complex with metal ion [17-18]. Some bands are appeared at between 600-400cm-1, these bands may be assigned to the M-O, and M-Cl stretching [19, 20].
Antibacterial activities
The antibacterial studies of synthesized compounds have been carried out with some bacterial microorganisms namely Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes having Ciprofloxacin as the standard antibiotic under the disc diffusion method (Figure 2, 3).
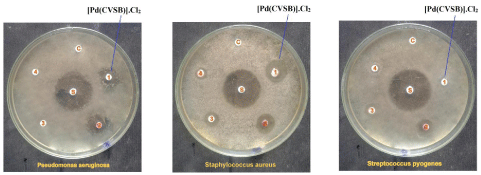
Figure 2: Antibacterial effects of Palladium (II) complex of Chitosan-vanillin
schiff base (CVSB).
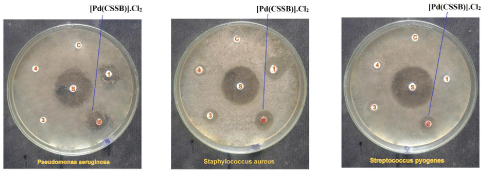
Figure 3: Antibacterial effects of Palladium (II) complex of Chitosansalicylaldehyde
schiff base (CSSB).
The Pseudomonas aeruginosa organism has been used for analyzing the capacity of the synthetic drug [Pd(CVSB)].Cl2 and [Pd(CSSB)].Cl2 with 17 units standard antibiotic Ciprofloxacin. The synthesized drugs were employed for antibacterial activity against bacterial pathogen Pseudomonas aeruginosa, the inhibitory activity of both drugs are observed as higher by its zone of inhibition. The zone of inhibition of both drugs is 11 mm units.
The Gram positive organism Staphylococcus aureus requires 19 parts of Ciprofloxacin to compare the efficacy of [Pd(CVSB)].Cl2 offers 11 mm of antibacterial activity but the [Pd(CSSB)].Cl2 complex goes to lesser its activity which has 10 mm units of inhibition against the same bacterial organism. It is found that the complexes are more active against two bacterial micro organisms such as Pseudomonas aeruginosa and Staphylococcus aureus.
A 20 units of Streptococcus pyogenes has been chosen for the standard antibiotic Ciprofloxacin for comparing the antimicrobial activity of [Pd(CVSB)].Cl2 and [Pd(CSSB)].Cl2 against Streptococcus pyogenes. Even though the inhibition is found to be greater under [Pd(CVSB)].Cl2 but [Pd(CVSB)].Cl2 is lesser effects. The zone of inhibition of the synthesized drugs is 07 mm and 09 mm units respectively against the same organism. The antibacterial results are furnished in Table 1.
Antibacterial activity data of Palladium (II) complexes (each 1000 mg/L)
S. No.
Bacteria
Standard Antibiotic Disk*
Zone of inhibition mm in diameter
[Pd(CVSB)].Cl2
[Pd(CSSB)].Cl2
Control
1
Pseudomonas aeruginosa
17
10
11
-
2
Staphylococcus aureus
19
11
10
-
3
Streptococcus pyogenes
20
07
09
-
*Ciprofloxacin.
Table 1: Antibacterial activity data of Palladium (II) complexes.[disc diffusion method].
Conclusion
The new series of chitosan schiff base Palladium (II) complexes were synthesized by the chemical reaction of chitosan with aldehydes. The antibacterial screening of the complexes and the ligands have been carried out against bacterial pathogen such as Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes using nutrient agar medium by disc diffusion method. The test solutions were prepared in DMSO. Generally, the Palladium (II) complexes have shown good antibacterial activity than the schiff base ligands against the same bacterial organisms under the identical experimental conditions. The results further support the fact that the Palladium (II) complexes with potential ligand can exaggerated the antibacterial activity increases with minimum concentration. The antibacterial effects of the synthesized chitosan schiff base complexes are more active against the bacterial pathogens. This is due the permeation process of the complex compounds. The metal ion of the complex compounds involved the permeation process to stop further growth of the bacterial organisms.
Acknowledgements
The authors thank to Research and Development Centre, Bharathiar University, Coimbatore for their guidance in fulfillment of our research and Dr. S. Chandrasekar Research centre, Karunya University, for their assistance.
References
- Hamdine M, Heuzey MC, Bégin A. Effect of organic and inorganic acids on concentrated chitosan solutions and gels. Int J Biol Macromol. 2005; 37: 134-142.
- Volesky B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hydro Metall. 2001; 59: 203 - 216.
- Alves NM, Mano JF. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol. 2008; 43: 401-414.
- Santos JE, Dockal ER, Cavalheiro ETG. Synthesis and Characterization of Schiff Bases from Chitosan and Salicylaldehyde Derivatives. Carbohydr. Polym. 2005; 60: 277-282.
- Kumar MNVR. A Review of Chitin and Chitosan Applications, Reactive and Functional Polymers. 2000; 46: 1-27.
- Muzzarelli RAA. Chitin, Pergamon Press. Oxford. 1977.
- Naveen A Anan, Showky M Hassan, Eman M Saad, Ian S Butler, Sahar I Mostafe. Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidenechitosan Schiff-base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohydr. Res. 2011; 346: 775-793.
- Vipin Bansal, Pramod Kumar Sharma, Nitin Sharma, Om Prakash Pal, Risnabha Malviya. Applications of Chitosan and Chitosan Derivatives in Drug Delivery. Adv. Biol. Res. 2011; 5: 28-37.
- Sashiwa H, Shigemasa Y, Roy R. Chemical modification of chitosan. Part 9: Reaction of N-carboxyethylchitosan methyl ester with diamines of acetal ending PAMAM dendrimers. Carbohydr. Polym. 2002; 47: 201-208.
- Bhuvaneshwari S, Sruthi D, Sivasubramanian V, Niranjana kalyani and Sugunabai J. Development and characterization of chitosan film. Int. J. Eng. Res. 1: 292-299.
- Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006; 114: 100-109.
- Chinnasamy Jayabalakrishnan, Ramasamy Karvembu, Karuppannan Natarajan. Catalytic and antimicrobial activities of new ruthenium(II) unsymmetrical Schiff base complexes. Transition Met. Chem. 2002; 27 790-794.
- Kupta KC, Alekha Kumar Sutar. Catalytic activities of Schiff base transition metal Complexes. Coord. Chem. Rev. 2008; 252: 1420-1450.
- Antony R, Theodore David S, Karuppasamy K, Saravanan K, Thanikaikarasan S, Balakumar S. Structural, Surface, Thermal and Catalytic Properties of Chitosan Supported Cu(II) Mixed Ligand Complex Materials. J.Surf. Eng. Mater. Adv. Technol. 2012; 2: 284-291
- Al-kubais AH, Ismail KZ. Nickel(I1) and palladium(I1) chelates of dehydroacetic acid Schiff bases derived from thiosemicarbazide and hydrazinecarbodithioate. Can. J. Chem. 1994; 72: 1785-1788.
- Selwin Joseyphus R, Justin Dhanaraj C, Sivasankaran Nair M. Synthesis and characterization of some Schiff base transition metal complexes derived from vanillin and L(+)alanine. Transition Met. Chem. 2006; 31: 699-702.
- ZHAO Guoliang, LI Liangchao, LIU Xinghai, ZHANG Pinghua. Synthesis, characterization and biological activity of transition metals with Schiff base derived from adamantaneamine and o-vanillin. Wuhan University J. Nat. Sci. 2006; 11: 427-431.
- Periyasamy Viswanathamurthi, Nallasamy Dharmaraj, Sankaran Anuradha, Karuppannan Natarajan. Ruthenium (III) complexes with tetradentate Schiff bases containing triphenylphosphine or triphenylarsine Transition Met. Chem. 1998; 23: 337-341.
- Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compound [M]. 4th ed. Willey & Sons. New York. 1986.
- Vinod K, Om P Pandey, Soumitra K. Sengupta. Synthesis and physico-chemical and biological studies on ruthenium (III) complexes with Schiff bases derived from aminocarboxylic acids. Transition Met. Chem. 1987; 12: 509-515.