
Research Article
J Bacteriol Mycol. 2016; 3(2): 1028.
Demographic, Clinical and Bacteriological Characteristics of Human Leptospirosis in Sri Lanka: A Retrospective Study
Karunanayake L1*, Karunanayake SAAP2, Perera KCR1, Senarath U3, Gunarathna HDNT1 and Peter D1
1National Reference Laboratory for Leptospirosis, Department of Bacteriology, Medical Research Institute, Colombo, Sri Lanka
2Department of Clinical Medicine, Faculty of Medicine, University of Colombo, Sri Lanka
3Department of Community Medicine, Faculty of Medicine, University of Colombo, Sri Lanka
*Corresponding author: Karunanayake L, Consultant Clinical Microbiologist, National Reference Laboratory for Leptospirosis, Department of Bacteriology, Medical Research Institute, Colombo, Sri Lanka
Received: June 13, 2016; Accepted: July 02, 2016; Published: July 06, 2016
Abstract
The clinical and laboratory diagnosis of human leptospirosis is complex due to a variety of factors, but the Microscopic Agglutination Test (MAT) is probably the most specific laboratory test. We describe the demographic, clinical and bacteriological features of all MAT-confirmed cases in 2015 at the National Reference Laboratory in Sri Lanka. The majority of cases had acquired the infection through indirect transmission through high-risk occupations, which relate to the lower socio-economic groups, and a majority was from the economically productive age group. A second sample during follow-up was useful. The common prevalence of serogroups such as Tarassovi and Autumnalis implicate the buffaloe as an important carrier animal in Sri Lanka. There was no significant association between organ involvement and province, except for multi-organ dysfunction syndrome. Several significant associations between serovar and organ involvement were found which could be useful for future pathophysiological studies. Serogroups Australis and Cyanopteri were found significantly more commonly in Uva Province. Awareness of the disease among high risk groups and bovine vaccination programs with locally prevalent serovars should be urgently considered for this potentially fatal disease.
Keywords: Leptospirosis; Serogroups; AKI; MAT; Sri Lanka
Abbreviations
AKI: Acute Kidney Injury; CFR: Case Fatality Rate; MAT: Microscopic Agglutination Test; MODS: Multi-Organ Dysfunction Syndrome; NHSL: National Hospital of Sri Lanka; NRL: National Reference Laboratory; SND: Standard Normal Distribution
Introduction
Leptospirosis is a neglected tropical zoonosis caused by pathogenic spirochetes of the species Leptospira interrogans. It is distributed worldwide and is endemic in Southeast Asia. It is maintained by chronic renal infection in carrier animals, which excrete leptospira in their urine, contaminating the environment [1].
Human leptospirosis is an acute febrile illness with a wide spectrum of clinical manifestations, ranging from mild to severe. The infection occurs through direct or indirect exposure to urine or tissue of infected animals. Direct contact is important in specific occupations such as veterinarians, abattoir workers and animal handlers. Indirect contact is more common, and is the mode of transmission in the great majority of cases, especially in tropics [1]. Such exposure occurs in a contaminated environment especially with wet soil and water, as in rice farming, flooding after heavy rainfall, and recreational water sports [2].
Leptospirosis is a notifiable diseasein Sri Lanka [2]. It is highly endemic, with an annual incidence rate of >10/100,000 population. The Case Fatality Rate (CFR) ranges from 1.5-2.9% [2]. In 2008, Sri Lanka reported the largest outbreak of leptospirosis yet, with 7,406 notifications of suspected cases and 204 deaths, with an annual incidence rate of 35.7/100,000 population. The CFR was 2.7% [2,3].
The agricultural economy especially rice farming, warm tropical climate, and seasonal rainfalls with flooding provide an ideal environment for its indirect transmission in Sri Lanka. There are two main paddy cultivation seasons in the country, named ‘Yala’ and ‘Maha’. Yala, the minor growing season in the dry zone, occurs with the first monsoon rains in March-April. The major growing season, Maha, begins with the second inter-monsoon rain in October to November [4].
The first case of clinically suspected Weil’s disease in Sri Lanka (Ceylon) was reported in 1953 [5]. In 1959, Rajasuriyaetal reported a case series of leptospirosis with laboratory confirmation [6]. The cases were serologically confirmed at National Reference Laboratory (NRL), Medical Research Institute by complement fixation test and later isolated by guinea pig inoculation, and identified as Leptospira Icterohaemorrhagiae [7]. In 1971, Nityananda and Harvey examined the different serotypes and their reservoir hosts in Sri Lanka. Their findings suggested the existence of a diversity of serotypes maintained by different maintenance hosts such as rodents, domestic farm animals and dogs [5]. Since then, many new reference leptospira serovars from humans and animals have been added from Sri Lanka to the world literature [5,8].
In 1962, a serological survey of occupational groups was done at NRL using macro-agglutination test. Rice field workers, sewer workers, workers in coconut plantations and desiccated coconut mills, sugar cane workers, abattoir workers, fish market workers and river bathers were identified as at risk [9].
In Sri Lanka, dengue fever and leptospirosis are significant public health problems. They occur throughout the year with peaks related to rainfall, and show similar clinical manifestations in early illness. In comparison to dengue, however, leptospirosis carries a higher risk of death in Sri Lanka. In the first quarter of 2015, 1,130 clinically suspected leptospirosis cases were notified with 23 deaths (CFR 2.03%). In contrast, for the same period 12,035 clinically suspected cases of dengue fever/dengue hemorrhagic fever were notified, with 27 deaths (CFR 0.22%), showing a10-fold higher risk of death for leptospirosis [10]. Severe leptospirosis involves renal, hepatic, pulmonary, cardiac and pancreatic complications, often in combination leading to Multi- Organ Dysfunction Syndrome (MODS).
The Microscopic Agglutination Test (MAT) is the serological reference method and the most common serological technique used for confirmation [1,11]. It detects IgM and IgG agglutinating antibodies. Microscopic agglutinating antibodies appear at the end of first week of illness and reach peak levels during third to fourth week [11,12]. It is a complex test to maintain, perform and interpret, and the highest specificity is achieved by using live cultures of different serogroups of leptospires. Therefore, the use of this test is restricted to reference laboratories. While it has high specificity, the sensitivity in early illness is low especially if used for single specimens [11].
Niloofa et al analyzed the accuracy of the MAT in 255 clinically diagnosed patients, with a single acute sample, in the NRL using only the saprophytic species (Patoc -1) as the antigen. The results showed a very low sensitivity of 55.3%, specificity of 95.7%, positive predictive value of 0.95 and negative predictive value of 0.55 with a single acute sample [13]. In the same study, assuming that all tests were imperfect using the Bayesian Latent Class Modelling for a single sample, the sensitivities of MAT, IgM ELISA and Lepto check were 77.4%, 87.4% and 86.0%, and the specificities were 97.6%, 82.9% and 84.5%, respectively [13]. Accordingly, the MAT using live organisms was confirmed as the ‘serological reference’ test in our setting.
Limmathurotsakul et al (2012) questioned the accuracy of the MAT as a reference test in Thailand. In that study the MAT was not performed in Thailand [14]. The country-specific diversity of serovars and strain differences may have contributed to their final analysis. Although there are many limitations, with a pathogenic panel carefully selected by local laboratory experts with their knowledge on local patterns and strains, the MAT claims to be the best serological reference test in this complex disease.
Furthermore, MAT is the most appropriate test for epidemiological sero-surveys [11]. MAT data is useful to determine the diverse serogroups within a country. In 2012, a preliminary serological study by MAT was done using 8 pathogenic serogroups. Interestingly, Pyrogenes was the commonest serogroup, followed by Pomona, Autumnalis and Icterohaemorrhagiae [15]. In the same period leptospira cultures were identified as belonging to Pyrogenes and Autumnalis serogroups (unpublished data) [16]. MAT with a live pathogenic panel was introduced in our laboratory from 2014,and periodically the panel was changed to increase the positivity of the test.
An animal species can be a maintenance host to one serovar and an incidental host to another [17]. In rural Sri Lanka, water buffaloes are still utilized for paddy cultivation. They are used in land preparation before the monsoon. Serological studies in buffaloes in Sri Lanka had shown the presence of serogroups Autumnalis, Sejroe, Pyrogenes and Tarassovi [18,19]. Another study done in Sri Lankan murid rodents in wild, urban and urban-wild interface has shown the presence of serogroups Pomona, Autumnalis, Sejroe, and Javanica, mainly in rats and mongoose species [20]. In a local sero-survey, unvaccinated dogs were found to be positive for serogroups Canicola, Australis, Icterohaemorrhagiae and Djasiman [21]. The knowledge of the prevalent serovars and their maintenance hosts is useful to understand the epidemiology of leptospirosis and to select targeted prevention and control strategies.
Our objective is to contribute towards the knowledge of the epidemiology, clinical manifestations and laboratory aspects of human leptospirosis in Sri Lanka. We have analyzed the findings at the NRL, which is the reference center that performs MAT and processes free of charge specimens from state hospitals throughout the country. We would discuss the background prevalence of leptospira serotypes in the provinces and its implications.
Material and Methods
A retrospective study was carried out at the NRL using data for 2015. The target population consisted of clinically suspected patients from all 9 provinces in Sri Lanka whose specimens yielded a significant result for leptospirosis antibodies at the NRL during 2015. Out of the total number of 2,662 clinical records received in the laboratory, only 2,433 non-repetitive, single records were used for the analysis of data. Of these, 1,004 (41.3%) were serologically confirmed as leptospirosis.
Laboratory confirmation
MAT positivity or a significant result was based on a cut-off titre of ≥ 1/320, seroconversion, or four-fold rise in paired sera in any one of the serovars in the panel. When analyzing multiple samples in the same patient, the results of the second sample were used. To assess the prevalence of serogroups, a cut-off titre of ≥ 1/40 by MAT was used.
For the present study 13 serovars were included. The pathogenic panel included 12 serogroups:Australis, Autumnalis, Bataviae, Canicola, Cynopteri, Grippotyphosa, Hebdomadis, Icterohaemorrhagiae, Pomona, Pyrogenes, Sejroe, Tarassovi. The saprophytic serogroup was Semaranga.
Information on disease severity
The analysis of clinical information, including disease severity, was based on the information in the request form. Clinical information had been determined by specialists in internal medicine in hospitals where the patients were treated.
The hospitals belonged to different levels of care such as Teaching Hospitals, Provincial General Hospitals and Base Hospitals. The National Hospital of Sri Lanka (NHSL) is the premier healthcare provider in the country. Therefore, severely ill patients with complications are transferred there. Also, it is a popular station for patients with renal impairment. This is evident in a previous study that showed that 67.7% of severe leptospirosis with Acute Kidney Injury (AKI) was treated in the Colombo group hospitals [22].
Statistical analysis
Socio-demographic data of the patients were summarized as means or percentages, as appropriate. The clinical and serological data were entered in binary form (present=1, absent=0) according to the defined criteria, and presented as percentages. The crosstabulations were generated to compare clinical and serological data across the provinces, with SND test used for statistical significance between proportions. The association between serovars and the organ involved in severe leptospirosis, and the association between serovars and province were determined by chi-square test. Data were analyzed using SPSS software. A p value <0.05 was considered statistically significant.
Results
In 2015, 4,402 clinically suspected leptospirosis cases were notified to the Epidemiology Unit [23] while the NRL received 2,433 specimens. Although it is not possible to make a direct comparison between notification numbers and laboratory-tested numbers as these do not refer to the same populations, it is noteworthy that the number of cases tested in 2015 constitutes over half (55%) the number of notifications.
Demographic and Laboratory results
Out of the total 2,433 non-repetitive records of clinically suspected cases of leptospirosis, 1,429 was negative and 1,004 (41.3%) yielded a significant result by MAT. For final analysis of demography and laboratory results, these 1,004 cases were analyzed.
Multiple samples were received in 179 patients (7.3%, n=2,433). In 106 patients the MAT titer was significantly higher in the second sample. Therefore, 59.2% of these patients were confirmed by MAT with the second sample.
The majority (78%) of the samples was received from wards and 19.2% from intensive care units. The rest (2.8%) were received from preliminary care units, dialysis units, clinics and postmortem samples. Fourteen (21%) postmortem blood samples were confirmed by MAT. Samples were received throughout the year. The number of samples was higher in March and in September to December compared to other months.
The distribution of cases according to province is shown in Table 1. The majority (n= 700, 69.7%) were from hospitals in Western Province and least from North Western Province (n=16, 1.6%). A majority were received from NHSL (32.7% n=328) and Colombo South Teaching Hospital (11.1% n=111).
Province
N
%
Central Province
18
1.8%
Eastern province
37
3.7%
North Central Province
60
6.0%
Northern Province
23
2.3%
North Western province
16
1.6%
Sabaragamuwa Province
29
2.9%
Southern Province
73
7.3%
Uva Province
48
4.8%
Western Province
700
69.7%
Table 1: Number and proportions of laboratory confirmed cases by province.
The male: female ratio was 5:1. The mean age was 42 years (range 5-70 years), with clustering in the age interval 30-59 years (621 cases, 61.8%). Occupation was not recorded in 736 cases. Out of the 268 recorded occupations, 66% (n=177) belonged to highrisk occupations such as paddy farmers, laborers including manual workers, and men in armed forces. No- or low-risk occupational groups included sedentary workers, including drivers (25.4%) and students (8.6%).
Prevalent serogroup/s
The common serogroup identified was Leptospira serogroup Tarassovi. The least common was Sejore (3.4%). The serogroups and the percentages are shown in Figure 1.
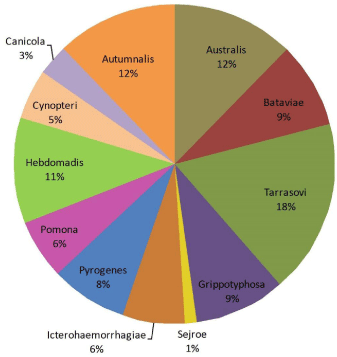
Figure 1: Proportions of serogroups in laboratory confirmed cases.
The association between serovar and province (see Table 2) was analyzed by the chi-square test. The serogroup Australis was significantly higher in Uva Province (66.7%) compared to North-central (30.0%), Northern (17.4%), North-western (12.5%), Sabaragamuwa (20.7%) and Western (31.7%) provinces. The serogroup Cynopteri was also significantly higher in Uva Province (39.6%) compared to Western Province (10.9%).
Serogroup
Province*
CP%
EP%
NCP%
NP%
NWP%
SABP%
SP%
UP%
WP%
Australis
27.8
45.9
30
17.4
12.5
20.7
39.7
66.7
31.7
Bataviae
11.1
21.6
18.6
26.1
12.5
17.2
19.2
31.3
24.6
Tarrasovi
90.9
100
96.7
100
100
93.8
94.4
92.9
98.2
Grippotyphosa
22.2
35.1
25
8.7
6.3
20.7
26
35.4
24.7
Sejroe
5.6
8.1
5
0
0
6.9
2.7
6.3
2.9
Icterohaemorrhagiae
11.1
27
16.7
8.7
43.8
17.2
11
25
16.3
Pyrogenes
16.7
24.3
16.7
4.3
6.3
13.8
15.1
6.3
24
Pomona
11.1
13.5
13.3
17.4
18.8
6.9
15.1
18.8
17.1
Hebdomadis
33.3
37.8
21.7
17.4
31.3
24.1
31.5
45.8
28
Cynopteri
16.7
13.5
21.7
4.3
12.5
6.9
21.9
39.6
10.9
Canicola
12.5
21.7
6.7
50
33.3
15.4
15.8
11.8
16.1
Autumnalis
16.7
35.1
33.3
17.4
18.8
17.2
42.5
45.8
32.9
*CP-Central Province, EP-Eastern Province, NCP-Northcentral Province, NP-Northern Province, NWP-Northwestern Province, SABP-Sabragamuwa Province, UP-Uva Province, WP-Western Province.
Table 2: Percentage of positive cases for each serogroup by province.
Clinical information
Out of 1,004 positive cases, only 56% (n=563) had reported clinical information. For clinical information only these 563 records were analyzed.
Exposure to risk factors were present in 43.5% (n=245). Fever was reported in 86.6% of patients. Other nonspecific symptoms reported included arthralgia, myalgia, conjunctival suffusion, red eye, headache, vomiting and diarrhea.
Table 3 shows the analysis of cases of severe leptospirosis according to the organ/s involved. The commonest organ involvement was Acute Kidney Injury (AKI), with 33.2% (n=187). Pulmonary involvement included Acute Respiratory Distress Syndrome (ARDS), pulmonary hemorrhage and pulmonary infiltrates. Although liver involvement (ranging from increased serum transaminases to hepatic encephalopathy) was noted in 97 cases, their common occurrence in alcoholic liver disease makes it difficult to attribute these directly to leptospirosis. Other complications noted occasionally included hemiplegia, hepatic encephalopathy, acute quadriplegia, cerebellar signs, Guillain-Barre syndrome, and skin rash.
Organ involved
%
N
Renal
37.80%
213
AKI
33.20%
187
Liver
17.20%
97
Pulmonary
7.60%
43
Pancreatitis
0.90%
5
Myocarditis
4.10%
23
Table 3: Presence of organ involvement in severe leptospirosis.
The association between serovar and organ involved in severe leptospirosis was analyzed by chi-square test. Significant associations were found for AKI and serovars Bataviae (p=0.003), Sejroe (p=0.047), Pyrogenes (p=0.006), Canicola (p=0.03), Autumnalis (p=0.045); for liver involvement, Pomona (p=0.036), Hebdomadis (p=0.003); and for pancreatitis, Australis (p=0.023), Bataviae (p=0.001), Sejroe (p=0.02), Icterohemorrhagiae (p=0.000), Pomona (p=0.000), Hebdomadis (p=0.000), Cynopteri (p=0.000) and Autumnalis (0.000).
The distribution of organ involvement in severe leptospirosis in relation to province was analyzed by comparing the respective proportions across the 9 provinces, together with statistical significance for the difference between proportions using SND test. Pancreatitis was reported only from the North-Central and Uva provinces. A statistically significant difference for the distribution of organ involved in severe leptospirosis according to province was found only for MODS, which was found to be significantly commoner in Northern Province (15.4%) compared to Western Province (1.3%).
Discussion
Leptospirosis has a high case fatality in Sri Lanka. The similarity of clinical manifestations with other common tropical infectious diseases, such as dengue, hanta virus disease and typhus, and extreme variations in the clinical manifestations, make vigilance by clinicians on this potentially fatal disease essential. Due to the complexities of the organism and the laboratory tests, laboratory diagnosis is difficult. We believe that the MAT offers the most specific test, when carefully done in a reference laboratory using live panels and paying attention to the locally prevalent pathogenic serogroups.
This paper describes the most current demographic, clinical and bacteriological aspects of leptospirosis cases confirmed by MAT at the NRL for 2015. This knowledge could be important for clinicians for early management of patients and for policymakers to customize preventive and control strategies in Sri Lanka.
In 2015, 4,402 clinically suspected leptospirosis cases with 71 deaths were notified to the Epidemiology Unit [23]. The incidence rate of notified leptospirosis for 2015 was 20.9/100,000 population, with a CFR of 1.6%. The highest numbers of cases were notified from Western, Sabaragamuwa and Southern Provinces [23].
In 2015, 2,433 specimens were received for serological testing at the NRL. The highest numbers of positive cases were from Western, Southern and Uva Provinces. Approximately 21% of deaths were confirmed by MAT.
A higher number of samples of clinically suspected patients were seen in March and September to December, corresponding with monsoon seasons for paddy cultivations in ‘Yala’ and ‘Maha’ respectively.
A majority (66%, n=177) of these patients belonged to high-risk occupations such as farmers, laborers, manual workers and men in forces. It is well known that a majority of our lower socioeconomic labor force often work part-time in paddy fields. About a quarter of patients (25.40%) are from no- or low-risk categories such as drivers and office workers. Their exposures would have been recreational such as bathing in rivers and lakes and/or part-time work in paddy fields. The diversity of occupations reiterates that the disease risk is related more towards contaminated environment than direct exposure.
In rural areas, children regularly bathe in rivers, and paddy fields and marshy lands are used as playgrounds. In this study 8.6% of patients were students. Previous serological studies have implicated river bathing and white water rafting as risk factors [24,25].
Our study shows the importance of obtaining a second sample for MAT testing, as 59.2% of patients were confirmed with a second sample. In this study, multiple samples were received only in 179 (7.3%) patients. Similar results were shown in another study [26].
In this study, the serogroups Tarassovi and semaranga together had confirmed 97.3% of cases by MAT and each of them had identified 83 and 11 more cases respectively. Interestingly, 2.4% of cases were serologically confirmed with other serogroups. This highlights the need for the selection of the most appropriate panel for MAT that can be used with ease. The use of all 25 serogroups in a panel for routine diagnosis is cumbersome.
The most common pathogenic serogroup in this study is Tarassovi, followed by Autumnalis, Australis, Hebdomadis and Grippotyphosa. The least common serogroup is Sejroe. The high prevalence of Sejroe serogroup in buffaloes and rodents with low prevalence in human infection is noteworthy. Theoretically, this can be due to shorter survival time in the environment, poor human adaptation or a good vaccination program against serogroup Sejroe. In Sri Lanka, only the canine vaccination against serogroups Canicola and Icterohaemorrhagiae is available. Therefore, the most likely reasons would be environmental survival of Leptospira serogroup Sejroe and/or poor human adaptation. Survival of pathogenic leptospires in the environment depends on many factors, including pH, temperature and presence of other inhibitory substances in the soil [1]. The environmental survival of Leptospira serovar hardjo studied in Malaysia showed the shortest survival time of 2 hours in undiluted cattle urine with direct sunlight and up to 6 days in loam soil in shaded areas. It showed that pH 7-8, low temperature and shade increase the survival time [27]. Soil type in paddy fields, direct sunlight with high tropical temperature, and less shady areas could be some factors resulting in short survival of these strains in our environment. This is an important area for future research.
According to available data in Sri Lanka, the important maintenance host animals for the highly prevalent serogroups (Tarassovi, Autumnalis, Australis, Hebdomadis and Grippotyphosa) in human leptospirosis is more likely to be buffaloes followed by dogs and rodents. Wijewardene et al. studied the seroprevalence of leptospirosis in buffaloes. The most prevalent serogroup was Autumnalis, commonly found in buffaloes from Badulla district (Uva Province) [28]. Interestingly, in our study the second commonest serogroup is Autumnalis (n=331), and its highest prevalence (45.8%) were recorded from Uva province. This highlights the need for collaborative research on animal hosts and serovars in humans to guide prevention and control strategies.
The lack of provision of clinical information when requesting laboratory investigations was evident in this study, with only 56% (563 of 1,004 records) giving any information. Therefore, our findings on clinical information are likely to be underestimates. Clinicians should be encouraged to give information more frequently, as retrospective analyses of sizeable databases such as ours will then yield more useful results and conclusions. In addition, clinical information is useful for accurate interpretation of test results and selecting further tests for analysis.
In our study the commonest organ involved in severe leptospirosis was AKI, which is not surprising since a large number of cases were from reference centers for renal replacement therapy. In addition, pulmonary, cardiac and pancreatic involvement, severe sepsis and MODS were also noted.
When seen in relation to serovar, significant associations were found between AKI and 5 serovars (Bataviae, Sejroe, Pyrogenes, Canicola and Autumnalis), liver involvement and 2 serovars (Pomona and Hebdomadis), and pancreatitis and 8 serovars (Australis, Autumnalis, Bataviae, Cynopteri, Hebdomadis, Icterohemorrhagiae, Pomona and Sejroe). These findings may be useful in future pathophysiological studies relating to virulence factors. Organ involvement in severe leptospirosis appears to be evenly distributed amongst provinces, except for MODS which was found significantly more commonly in Northern Province compared to Western Province.
A significant association between serovar and province was found for Australis and Cynopteri, both of which were found in a significantly higher proportion in Uva Province compared to some other provinces: 5 provinces in the case of the former and 1 in the case of the latter. This may reflect an important carrier animal species in Uva Province. Further studies of animals known to transmit leptospirosis may be warranted in Uva Province, since if such a species carrying either or both of these serovars is identified, it may help customize preventive strategies in Uva Province.
Our study as well as previous studies [22] has shown high CFR of leptospirosis, as well as the fact that it affects the economically most productive age group, and especially farmers (rural Sri Lankahas a predominantly agriculture-based economy). This highlights the need for urgent attention to embark on prevention. Early diagnosis, antibiotic treatment and appropriate management of severe leptospirosis are important aspects in reducing the CFR.
The presence of a wide array of serovars and many different maintenance hosts make leptospirosis eradication difficult. The identification of maintenance hosts, which comprises the reservoir of infection, would prove useful to adopt targeted preventive and control measures. Also, in an outbreak, identification of the serovars is important in targeting specific control measures.
Awareness programs on the usefulness of protective clothing especially during paddy cultivation, vaccination of cattle and buffaloes, cleanliness of the environment without rodents are some aspects that should be considered. The maintenance host may differ between geographical areas, making it necessary to customize preventive measures by area. The coordination between the administrations of animal health and human health is extremely useful for the control of leptospirosis in the country.
Conclusion
Our study describes the demographic, clinical and bacteriological features of laboratory-confirmed cases of leptospirosis in Sri Lanka in 2015. The test used for confirmation was the MAT with a live pathogenic panel that had been selected using local experience at the NRL.
The vast majority of cases had acquired leptospirosis through indirect transmission from contaminated environments, determined by high-risk exposures, mostly occupational (paddy farming, manual labor, armed forces), but probably also recreational. The majority of cases were from the economically productive age group, and most exposure risks related to work done by the rural, lower socioeconomic groups and the armed forces. These highlight the urgent need for preventive and control efforts focused on these circumstances and population.
The full spectrum of clinical manifestations and complications was seen. There was no significant association between organ involvement or complication and province, apart from MODS, which was significantly commoner in Northern Province compared to Western Province. The common occurrence of renal involvement and AKI in Western Province is probably partly due to referral bias. Clinicians need to be made aware of all the manifestations of the disease, not merely the common ones. The usefulness of providing clinical information to the laboratory and the value of a second sample should be reiterated to them. We were able to identify several significant associations between serovars and specific organ involvements, which may be useful in future studies on pathophysiology, especially virulence and pathogenesis.
Available data suggests buffaloes as the main animal reservoir for leptospirosis in Sri Lanka. Identification of prevalent serovars in human and animal leptospirosis is important when implementing vaccination programs to select the appropriate vaccines.
We also noted that serogroups Australis and cyanopteri are seen significantly more commonly in Uva Province compared to several other provinces, pointing to the possibility of a special carrier animal species. Further animal studies in Uva Province may help identify it, and that may help to customize preventive strategies in that province.
Acknowledgement
Dr. P. Vijayachari, Director, Regional Medical Research Centre for Leptospirosis, Andaman Island, India and WHO Country Office - Sri Lanka are gratefully acknowledged for their unstinted guidance and support for the laboratory work.
References
- Levett PN, Haake IDA. Leptospira Species (Leptospirosis). Mandell GL, Bennett JE, Dolin R, editors. In: Mandell, Douglas and Bennett’s Principles and Practice of infectious diseases 7th edition Churchill Livingstone Elsevier. 2010; 3059 –3065.
- Karunanayake L. Human Leptospirosis: Microbiologist’s perspective. In: MRI Research Day 2012 Scientific Sessions. 2012; 69-76.
- Epidemiology unit, Ministry of Health, Sri Lanka, Surveillance report on leptospirosis. Epidemiological Bulletin Sri Lanka, 4th Quarter. 2010; 51: 18.
- Department of Agriculture, Sri Lanka. Climate of rice growing regions in Sri Lanka.
- Nityananda K, Harvey T. Leptospirosis in Ceylon – Epidemiological and Laboratory studies. Ceylon Journal of Medical Sciences. 1971; 20: 5-14.
- Rajasuriya K, somasunderam M, Nagaratnam N. Leptospirosis in Ceylon. J Trop Med Hyg. 1959; 62: 205-210.
- Nityananda K. Isolation of Leptospira in Ceylon. Ceylon Medical Journal. 1962; 7: 95-96.
- Nityananda K. A new serotype in the serogroup Icterohaemorrhagiae from Ceylon. Ceylon Journal of Medical Sciences. 1972; 71: 9-13.
- Nityananda K. Leptospirosis--serological survey of occupational groups in Ceylon. J Trop Med Hyg. 1967; 70: 250-254.
- Epidemiology unit, Ministry of Health, Sri Lanka, Surveillance report on leptospirosis, dengue. Epidemiological Bulletin Sri Lanka, 1st Quarter 2015.
- Vijayachari P, Sharma S. Natarajaseenivasan K. Laboratory diagnosis. In: Leptospirosis Laboratory Manual WHO. 2007; 27-45.
- Winn W, Allen S. Janda W, Koneman E, Procop G, Schreckenberger P, Woods G. Spirochetal Infections. Koneman’s Colour Atlas and Text book of Diagnostic Microbiology 6th edition Lippincott Williams & Wilkins 2006;1125-1150.
- Niloofa MJR, Fernando GTG, De Silva NL, Karunanayake L, Wickramasinghe H. Dikmadugoda N, et al. Diagnosis of Leptospirosis: Comparison between Microscopic Agglutination Test, IgM-ELISA and IgM Rapid Immunochromatography Test. PLoS ONE. 2015:10.
- Limmathurotsakul D, Turner EL, Wuthiekanun V, Thaipadungpaint J, Suputtamongkol Y, Chierakul W, et al. Fool’s Gold: Why imperfect reference tests are undermining the evaluation of novel diagnostics: A reevaluation of 5 diagnsotic tests for leptospirosis. In; Clinical Infectious Disease 2012: 55; 322-331.
- Karunanayake L, Perera R, Gunaratne N, Samaranayake A. A Preliminary Study on Serological Characterization of Human Leptospirosis in Sri Lanka In MRI Research Day 2012, Abstract and Programme book 2012;47.
- Unpublished data. National Reference Laboratory for Leptospirosis, MRI, Sri Lanka.
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001; 14: 296-326.
- Priyantha MAR, Gunawardana GA, Puvanendran S, Wijemuni MI, Alwis PS. Serological detection of leptosiraserovars from aborted buffaloes in Sri Lanka. In: Infectious Diseases. 480-483.
- Thammitiyagoda MG, Dissanayake HMRK, ChandrasiriP, Puvanendiran S, Jayathunga S. Identification of serovars of leptospira among buffaloes and buffalo farmers in Homagama veterinary division in Sri Lanka. In; Sri Lanka Veterinary Journal 2007:7-10.
- Jayamanne NMM, Gunawardene GAG, Dayawansa PN, Premawansa S. Sero-prevelance for Leptospira spp. In murid rodents with relation to wild, urban and urban-wild interface habitats in the wet zone of Sri Lanka. In; Proceedings of the 4th Biennial Academic Sessions of Allergy and Immunology Society of Sri Lanka 2009: 23.
- Thammitiyagoda MG, Somaratne P, Perera KCR, Priyantha MAR. Apreliminary study of the seroprevalence of leptospirosis in domestic dogs in suburbs of Colombo, Sri Lanka. In: Abstracts of Scientific Sessions, Sri Lanka Veterinary Association, 65th Annual Convention 14th June 2013.
- Rajapakse S, Weeratunga P, Niloofa MJR, Fernando N, Rodrigo C, Maduranga S et al. Clinical and laboratory associations of severity in a Sri Lankan cohort of patients with serologically confirmed leptospirosis: a prospective study. In; Tropical Medicine and Hygiene. 2015: 109: 710-716.
- Epidemiology Unit, Ministry of Health.
- Karunanayake SAAP, Wijesiriwardene B. Leptospirosis. Journal of the Ceylon College of Physicians 1999; 32: 24-29.
- Agampodi SB, Karunarathena D, Jayathilake N, Rathnayake H, Agampodi TC, Karunanayake L. Outbreak of leptospirosis after white-water rafting: sign of a shift from rural to recreational leptospirosis in Sri Lanka?. In; Epidemiology of Infection. 2013: 1-4
- Niloofa MJR, Fernando GTG, Thavarajah WRC, Karunanayake L, De Silva HJ, et al. Diagnosis of Leptospirosis: Confirmed vs. Probabale cases of leptospirosis and the importance of testing paired serum samples. In; MRI Research Day 2012 Scientific Sessions. 2012; 21
- Khairani-Bejo S, Bahaman AR, Zamri-Saad, Mutalib. The survival of LeptospiraInterrogansSerovarhadjo in the Malaysian environment. Journal of Animal and Veterinary Advances 2004; 3:123-129.
- Wijewardene TG, Wijewardene BDR, Appuhamy WNDGS, Premaratne KRVPM. Prevalence of leptospiral antibodies in buffaloes in Sri Lanka. In; Proceedings of the SAREC/NARESA Regional symposium on the role of buffaloes in rural development in Asia, NARES Press Colombo Sri Lanka. 1999: 415-426.