
Research Article
J Bacteriol Mycol. 2017; 4(1): 1045.
Purification and Characterization of Linamarase from Lactobacillus Plantarum
Adeleke BS*, Akinyele BJ and Olaniyi OO
Department of Microbiology, Federal University of Technology, Akure, Ondo State, Nigeria
*Corresponding author: Adeleke BS, Department of Microbiology, Federal University of Technology, Akure, Ondo State, Nigeria
Received: January 18, 2017; Accepted: March 07, 2017; Published: March 13, 2017
Abstract
In this study, bacteria isolated from fermenting bitter cassava peels were harnessed for linamarase production using standard microbiological procedures. Out of all the screened bacterial isolates, Lactobacillus plantarum exhibited highest linamarase activity and specific activity of 239.65μmol/min/ml and 43.57μmol/min/mg respectively. The linamarase was purified using DEAE Sephadex A-50 ion exchange chromatography and Sephadex G-150 column size exclusion or gel filtration chromatograpghy. A pH 6.0 was optimum for purified linamarase activity and relatively stable between 100 and 120 minutes. At 50oC, optimum temperature was reached and linamarase activity maintained at 65% temperature stability between 40 to 60 minutes. The incubation of linamarase enzyme with Ca and Mg ions enhanced linamarase activity, while Zn, Fe and Cu ions caused linamarase inhibition. The fact that purified linamarase from Lb. planatrum was active at moderate temperatures and wide range of pH can suggests its applications as detoxifying agent in food industries for animal feed formulation where extreme conditions are required.
Keywords: Lactobacillus plantarum; Linamarase activity; pH; Temperature stability
Introduction
Linamarin is a pro-carcinogenic substance found mostly in the leaves, peels and roots of some plants such as cassava, lima beans, flax etcetera in the family compositae, euphorbiaceae, linaceae, papaveraceae and fabaceae [1]. The presence of this toxic substance in some edible parts of plants limit its use as common source of foods to man. An insufficient food processing on consumption of underprocessed cassava roots containing linamarin above the safe level can result in upper motor neuron disease known as Konzo [2]. The toxicity is believed to be induced on ingestion of acetone cyanohydrin, the product from breakdown of linamarin. Hydrogen cyanide can be produced by hydrolytic reaction catalyzed by one or more enzymes from the plants containing cyanogenic glycosides when seeds are crushed, macerated, cut or mashed by traditional method [3]. The activities of linamarase-producing microorganisms in fermentation medium has contributed to the increase in the nutritive value, aroma, prolong shelf life, palatability of such product thereby ensuring food safety [4]. The traditional methods such as blanching and other methods have also contributed to the improvement in the palatability of such product thereby inhibiting the enzymatic reaction and prolong food shelf life.
Linamarase (EC.3.2.1.21) is a linamarin degrading enzyme, which on hydrolysis breaks down the glycolytic bond between β-glucose molecule and the chiral carbon atom linked to the nitrile group of linamarin present in cassava [5]. The application of this enzyme is limited because it is sensitive to environmental factors such as pH and temperature [6]. Over the years, humans have realized that selective propagation of animals and plants of desirable traits can improve and increase enzyme production in term of yield and quality. Beta- glucosidases - a derivative of linamarase from microorganisms can be more effective in detoxification of cyanogenic glycosides present in some plants [7]. The objectives of the study targeted the mechanism action of purified linamarase from Lb. plantarum for detoxification of linamarin present in cassava peels for industrial use.
Materials and Methods
Bacterial isolate
The bacterial isolate employed in this study was isolated from fermented cassava peels. The fermentation process was carried out for 96 hours in the Microbiology laboratory, Federal University of Technology, Akure, Nigeria. The fermentation of cassava peels account for the different bacteria isolated under cultural condition at room temperature.
Linamarase assay
Linamarase activity was assayed from the supernatant collected at the end of cold centrifugation using spectrophotometer (Laboratory Technology Digital Colorimeter). Linamarase activity was assay in the reaction mixture containing 0.5mL of 50mM potassium phosphate buffer pH 6.0 and 0.5ml precipitate of the crude extract containing substrate, incubated at 37°C for 15minutes and measured at 540nm wave lengths [8]. One unit of linamarase activity was defined as amount of enzyme producing 1 micromole of para-nitrophenol per minute under the experimental conditions as stipulated by Olaniyi et al. [8].
Protein determination
The protein estimation of the crude extract was determined by Bradford method and Bovine Serum Albumin (BSA) was used as standard [9].
Linamarase purification
The supernatant obtained after centrifugation was purified in three stages. In stage one, 100mL of the supernatant of crude linamarase obtained from the cold centrifugation was precipitated by adding 571g ammonium sulphate to obtained 70% ammonium sulphate concentration. After cold centrifugation at 6000rpm for 30minutes, the precipitate obtained was diluted in 100ml phosphate buffer (pH 6.0) and then dialyzed in the same buffer system. In stage two, the partially purified linamarase obtained after dialysis was purified on ion exchange column chromatography containing DEAE (diethylamineethyl) Sephadex A-50 (2.0 x 2.5 cm, Pharmacia). The eluates of the partially purified linamarase obtained from stage two were washed with 300mL ion-free water, followed by 200mL 0.01M Tris-HCl buffer pH 8.0. The gel was eluted with NaCl. The absorbance of each of the fraction was measured. In stage three, 2.5mL of partially purified linamarase was loaded onto a column chromatography (2.5 in diameter and 30 cm high) using Sephadex G-150 (Pharmacia). Phosphate elution buffer at 50mM pH 6.0 was applied with the flow rate 20ml/hr. A fraction of 5.0mL was collected at interval of 30minutes and the highest linamarase activity was determined with Para nitrophenyl β-D glucosides as a substrate, at 280nm (Jenwey 6305). Fractions with linamarase activity were pooled and concentrated in glycerol solution at 30oC [10,11].
Characterization of Purified Linamarase
Effect of pH on linamarase activity
Different buffer system 50mM of Glycine-HCl, pH 3.0, acetate buffer pH 4.0, phosphate buffer pH 6.0, Tris-HCl pH 8.0 were prepared. Each of this buffer solution was used to prepare 1% Para nitrophenyl β-D glucosides solution used as substrate in assaying the enzyme. The assay was carried out according to the standard assay procedure [11].
Effect of pH stability on linamarase
To observe the pH stability, the purified enzyme was kept in different pH buffers ranged from 4.0 to 12.0 at room temperature for different time interval up to 2 hours. Thereafter, relative linamarase activity was assay.
Effect of temperature on linamarase activity and stability
Linamarase activity was assay by incubating the enzyme reaction mixture at different temperature, 10 to 100°C for 30minutes. Thermostability of the enzyme was also determined. Samples were collected at 10minutes and thereafter residual activities were evaluated under standard assay conditions.
Effect of substrate concentration
The effect of substrate concentration [S] on the rate of enzyme action was studied. The substrate concentration [S] value ranged from 0.25mg/mL to 10.00mg/ml. The Lineweaver-Burk plot was made to determine the maximum velocity (Vmax) and Michaelis-Menten constant (Km). Both the maximum velocity (Vmax) and Michaelis- Menten constant (Km) of the enzyme were calculated.
Effect of metal ions on linamarase activity
A stock solution of 10mM for different metal (Ca, Mg, Zn, Fe and Cu) ions was prepared. Two milliliters of the prepared solution was mixed with 2ml of enzyme solution. The mixture was incubated for 5 minutes at room temperature. Zero point five milliliters (0.5mL) of the mixture were collected and linamarase assay was carried out according to the standard assay procedure.
Results and Discussions
Summary of purification
Table 1 shows the linamarase purification processes. The linamarase purification was obtained in three steps with approximately total protein recovery of 29% and 43.57 μmol/min/mg specific activity. The elution pattern of the linamarase enzyme on Sephadex A-50 gel filtration chromatography is shown in Figure 1. Linamarase activity was detected in fraction tubes 10.00 to 36.00. The fractionation tubes with linamarase activity were pooled and further purified on Sephadex G-150 (Figure 2). The variation in the linamarase activity peaks might be due to the rate of elution and surrounding conditions [12].
Step
Vol.
PC
EA
TP
TA
SPA
Yield
Fold
Crude Enzyme
500
37.95
56.28
18975
28140
1.48
100
1
Ammonium
sulphate precipitation
100
35.68
73.49
3568
7349
2.06
15.45
1.39
Ion exchange
Chromatography
45
6.81
212.36
306.45
9556.20
31.18
33.96
21.03
Gel
33
5.50
239.65
181.50
7908.45
43.57
28.10
29.38
Key: PC: Protein Content (mg/mL); SPA: Specific Activity (μmol/min/mg); EA: Enzyme Activity (μmol/min/mg); TA: Total Activity (μmol/min/mL); TP: Total Protein (mg).
Table 1: Summary of linamarase purification.
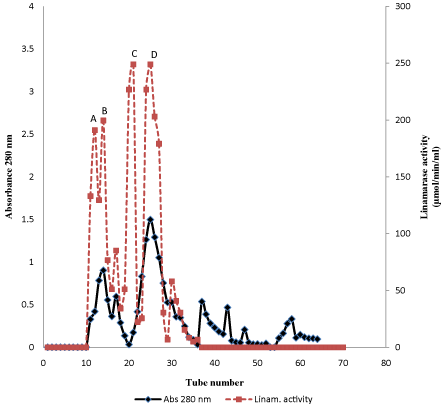
Figure 1: Elution profile of crude linamarase on DEAE Sephadex A-50.
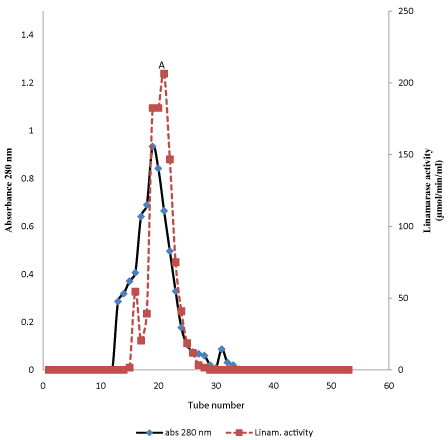
Figure 2: Elution profile of partially purified linamarase on Sephadex G-150.
Characterization of linamarase
Effect of temperature on linamarase acivity and stability: The effect of temperature on linamarase activity and stability is shown in Figures 3 and 4. The linamarase activity increased with increase in incubation temperature until an optimum percentage relative activity was reached at 50°C (Figure 3). An increase in temperature beyond the optimum has been reported to disrupt the enzyme structure and loss of activity. The result obtained from this study was in agreement with the findings of Ogbonaya and Florence [13] who reported temperature 50°C for linamarase activity from Lactobacillus delbrueckii NNRL B-763.
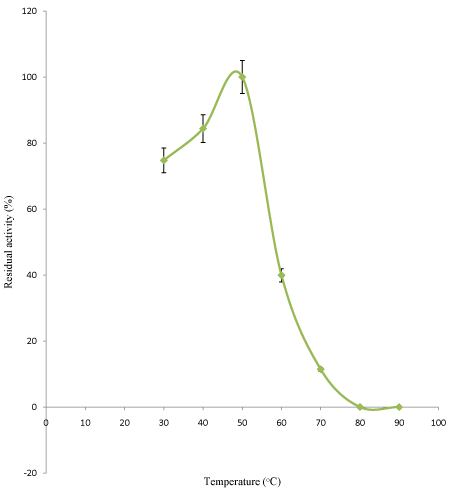
Figure 3: Effect of temperature on purified linamarase activity.
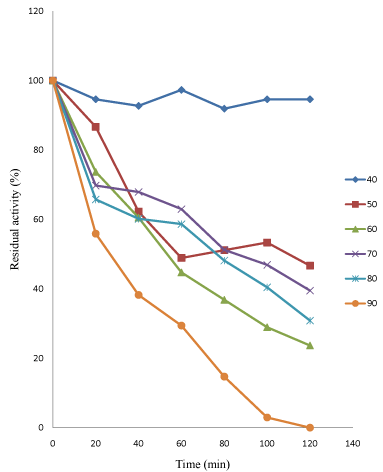
Figure 4: Thermostability profile of purified linamarase.
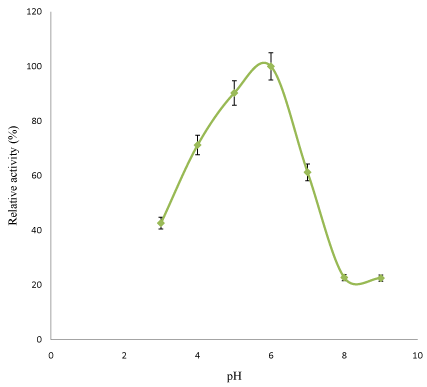
Figure 5: Effect of pH on purified linamarase activity.
The linamarase activity has the highest relatively temperature stability at 40°C between 20 and 40 minutes before a slight decline in its activity was observed. The linamarase activity has the lowest temperature stability at 80°C between 40 and 60 minutes (Figure 4). The enzymatic activity gradually decrease with increase in temperature. The result obtained was similar to the findings of Gueguen et al. [14] who reported optimum stability 40 and 50°C of β-glucosidase from Leuconostoc mesenteroides.
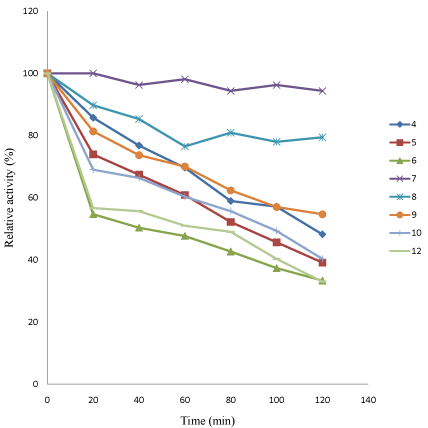
Figure 6: Effect of pH on stability of linamarase activity.
Effect of substrate concentration: The Michaelis constant (Km) indicated the concentration of substrate to fill the half active sites of an enzyme. It is also the measure of strength of the Enzyme- Substrate (ES) complex. The result obtained from this study showed higher substrate concentration. The purified linamaras exhibited Km value 2.27mg/mL and maximum velocity (Vmax) was 294.12μmol/ min/mL (Figure 7). Akinyele et al. [16] reported that high Km value indicates weak binding of the enzyme- substrate and vice versa. Therefore, linamarase with high Km value might have less affinity to the substrates [17].
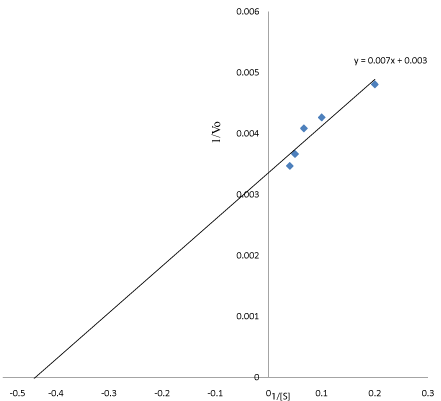
Figure 7: Line Weaver-Bulk double reciprocal plot (Km=2.27) mg/mL, Vmax=
294.12 μmol/min/mL).
Effect of metal ions on linamarase activity: The concentration of metal ions used in this study exerts their effect on linamarase activity (Figure 8). The metal ions exerted activation and inhibition on the linamarase activity. The linamarase activity was stimulated by Ca and Mg ions correspondingly, while Cu, Zn and Fe ions exhibited varied degree of inhibition on linamarase activity.
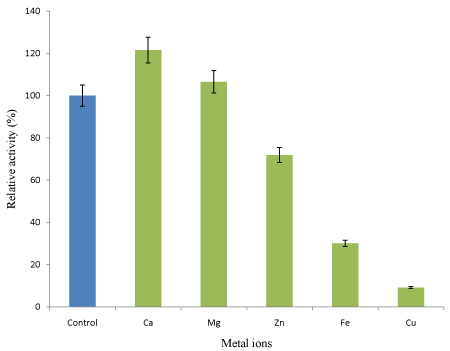
Figure 8: Effect of metal ions on linamarase activity.
Conclusion
The results obtained from this study revealed that purified linamarase was active over a wide range of pH and moderate temperature. Its stability suggests its utilization on introduction during fermentation of cassava peels for total detoxification process based on lactic acid fermentation in food industry where extreme conditions are required.
References
- Yessoufou A, Ategbo JM, Girard A, Prost J, Dramane KL, Moutairou K, et al. Cassava-enriched diet is not diabetogenic rather it aggravates diabetes in rats. Fundamental Clinical Pharmacology. 2002; 20: 579-586.
- Nzwalo H, Cliff J. Konzo: from poverty, cassava and cyanigen intake to toxic nutritional neurological diseases. Tropical Disease. 2011; 5: 1051-1371.
- Adetunji AR, Isadare DA, Akinluwade KJ, Adewoye OO. Waste-to-wealth applications of cassava: A review study of industrial and agricultural applications. Advances in Research. 2015; 4: 212-229.
- Azam-Ali S, Judge E, Fellows P, Battcock M. Small-scale food processing. A directory of equipment and methods. 2nd Edition. ITDG Publishing. 2003.
- Nwokoro O, Anya FO. Linamarase enzyme from Lactobacillus delbrueckii NRRL B-763: Purification and some properties of a β-glucosidase. Journal of Mexican Chemistry and Sociology. 2011; 55: 246-250.
- Nok JN, Ikediobi CO. Some properties of linamarase from cassava (Manihot esculenta Crantz) cortex. Journal of Food Biochemistry. 1999; 14: 477-489.
- Julius KI, Charles CA, James OA. Kinetic activity of commercial native linamarase (CNLIN) and engineered (β-glucosidase) from Saccharomyces cerevisiae on cassava linamarin. Advanced Journal of Food Science and Technology. 2014; 6: 149-154.
- Ogundu EC, Okoh PN, Ikediobi CO. Enzyme-Substrate specificity of β- D-glucosidase from fungal, bacterial and cassava sources using p-nitrophenol β-D-glucoside and cassava linamarin as substrates. Nature and Science. 2014; 12: 162-167.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976; 72: 248-254.
- Hammami I, Siala R, Jridi M, Ktari N, Nasri M, Mohamedali T. Partial purification andcharacterization of chiIO8, a novel antifungal chitinase produced by Bacillus cereus. Journal of Applied Microbiology. 2013; 115: 358-366.
- Olaniyi OO, Arotupin DJ, Akinyele BJ, Bamidele OS. Kinetics properties of purified β-mannanase from Penicillim itaculum. British Microbiology Research Journal. 2014; 2: 1092-1104.
- Olaniyi OO, Igbe FO, Ekundayo TC. Optimization studies on mannanase production by Trichosporonoides oedocephalis in submerged state fermentation. E3 Journal Biotechnology. 2013; 4: 110-116.
- Ogbonnaya N, Florence OA. Linamarase enzyme from Lactobacillus delbrueckii NRRL B-763: purification and some properties of a β-Glucosidase. Journal of Mexican Chemistry and Sociology. 2011; 55: 246-250.
- Gueguen Y, Chemardin P, Labrot P, Arnaud A, Galzy PJ. Purification and characterization of an intracellular β-glucosidase from a new strain of Leuconostoc mesenteroides isolated from cassava. Applied Journal of Microbiology. 1997; 82: 469-476.
- Brauman A, Keleke S, Malonga M, Miambi E, Ampe F. Microbiological and biochemical characterization of cassava retting, traditional lactic acid fermentation for foo-foo (cassava flour) production. Applied Environmental Microbiology. 1996; 62: 854-858.
- Akinyele BJ, Olaniyi OO, Adetunji CO. Screening and optimization of nutritional conditions for mannanase production by Penicillium italicum LAD-A5 in solid state fermentation. E3 Journal of Biotechnology and Pharmaceutical Reasearch. 2013; 4: 35-41.
- José MP, Barbosa RL, Souza C, Moura de Melo AT, Fricks CMF, et al. Biochemical characterization of lipase from a new strain of Bacillus sp. ITP-001. Quim. Nova. 2012; 35: 1173-1178.