
Research Article
J Bacteriol Mycol. 2017; 4(2): 1050.
A Brazilian Bentonite Previously Treated with Benzalkonium Chloride Reduces Pseudomonas Aeruginosa Growth
Nones J¹*, Savi GD², Müller L¹, Trentin AG³, Angioletto E², Soares C¹, Riella HG¹ and Nones J³
¹Department of Chemical Engineering, Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil
²Post-Graduate Program in Materials Science and Engineering, UNESC, Criciúma, Santa Catarina, Brazil
³Department of Cell Biology, Embryology and Genetics, Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil
*Corresponding author: Jader Nones, Department of Cell Biology, Embryology and Genetics, Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil
Received: April 19, 2017; Accepted: May 05, 2017; Published: May 12, 2017
Abstract
Pseudomonas aeruginosa can pose a serious threat to public health, as it causes a wide variety of clinical syndromes, including sepsis, pneumonia, meningitis, conjunctivitis and skin infections. Since early times, various types of bentonites have been used in the traditional medicine of most countries to avoid inflammatory reactions caused by different bacteria. Therefore, the primary objective of this work was to study the effects of natural bentonite and bentonite previously treated with an organic salt (benzalkonium chloride - BAC302) on the in vitro growth of Pseudomonas aeruginosa. For this purpose, after the organophilic treatment, we have previously characterized these bentonites (natural and BAC302) using Scanning Electron Microscopy (SEM) and the procedures by Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH). After characterization, the bentonite samples were submitted to the agar dilution method and the disc diffusion test to evaluate the effect on the growth of Pseudomonas aeruginosa. Our results show no differences in the surface morphology of the two materials tested (natural Brazilian bentonite and BAC302). However, the organic treatment reduces the specific surface area and increases pore diameter of BAC302. The growth of Pseudomonas aeruginosa was found to be reduced after treatment with BAC302, but not after treatment with natural bentonite. Another aim of the present work was to observe the effects of natural bentonite and BAC302 on fibroblast cultures. The two materials were not found to be toxic to fibroblast cells when dosed at the concentrations of 0.3-0.9 mg/ mL. This study contributes to a better understanding of the effects of bentonite on mammalian tissue, and the results may contribute to development of novel treatment strategies for P. aeruginosa infections.
Keywords: Bentonite; Benzalkonium chloride; Fibroblasts; Pseudomonas aeruginosa
Introduction
Pseudomonas aeruginosa is a nonfermentative, gram-negative bacterium responsible for a wide variety of diseases, including sepsis, pneumonia, meningitis, conjunctivitis, respiratory infections, dermatitis and soft tissue infections [1,2,3]. Moreover, it has been associated with both environmental reservoirs and healthcare workers’ carriage [4,5].
Despite the availability of antibiotics, P. aeruginosa infections continue to represent a public health threat. In the USA, the Centers for Disease Control and Prevention have reported 1.7 million hospital acquired infections yearly caused by all types of gram-positive (G+) and gram-negative (G-) bacteria, including Pseudomonas, resulting in 99,000 deaths every year [6].
As an aggravating factor, Pseudomonas aeruginosa is a notoriously - resistant bacterium that is increasingly refractory to antimicrobial chemotherapy [7,8,9]. Also, resistance to multiple classes of antimicrobials (multidrug resistance) increases in this organism. This can be caused by chromosomal mutations and/ or the acquisition of resistance genes via horizontal gene transfer [8,10,11]. In order to control this bacterium, alternative, safe and cost effective treatments should be developed. Compounds prepared with clay, such as bentonites, are attracting great attention in the field of biology and medicine because of the synergies found between its biopharmaceutical and technological features [12,13,14]. Bentonites have been shown to protect mammal cells from several insults [15,16,17]. Kevadiya et al. [18], for example, showed that clay minerals reduce genotoxic effects and facilitate drug delivery. In addition, various types of clay minerals have been used in the traditional medicine of most countries in order to treat a variety of diseases, mainly related to skin problems [19,20,21].
Alkylammonium cations, including Benzalkonium Chloride (BAC), is one of the most important quaternary ammonium compounds used for disinfection of surfaces in medical care applications as well as in the food and glue industries, because of their antibacterial activity [2,22,23,24]. Traditional pharmaceutical nasal sprays and drops require preservatives that prevent microbial contamination, and BAC is by far one of the most used preservatives in aqueous formulations [25,26] to prevent different infections.
We have recently demonstrated that natural bentonite and bentonite previously treated with benzalkonium chloride (BAC302) increase aflatoxin B1 adsorption significantly compared with untreated groups [17]. However, knowledge on the effects of bentonites associated with surfactants (BAC302) on cells or cell cultures is scarce [17,27]. Moreover, to our knowledge, there are no data reported about in vitro studies on the effects of organo-modified bentonite (BAC) on Pseudomonas aeruginosa bacteria.
This paper is the first report to describe the Pseudomonas aeruginosa bactericide effects of a Brazilian bentonite previously treated with organic salt (BAC302). Furthermore, it reports the results from toxicity studies on organic bentonites (BAC302) using fibroblast cells as a mammal culture model in order to test the biosafety of the product created, when applied to cells that form the epithelial tissue.
Materials and Methods
Reagents and chemicals
Dulbecco’s Modified Eagle Medium (DMEM-F12), penicillin, and streptomycin were purchased from Invitrogen. Fetal Bovine Serum (FBS) and trypan blue solution were purchased from Lonza (Verviers, Belgium). Dimethyl sulfoxide (DMSO), 4’,6-diamidino- 2-phenylindole dihydrochloride (DAPI) and benzalkonium chloride were purchased from Sigma–Aldrich (St. Louis, MO, USA). The materials for antimicrobial assays culture medium Mueller Hinton (MH) agar, Tryptic Soy Broth (TSB) and Brain Heart Infusion (BHI) were supplied by Himedia Laboratories (Bhaveshwar Plaza, Mumbai, India). Pseudomonas aeruginosa (ATCC 27853) was purchased from Newprov Products for Laboratory (Pinhais, Paraná, Brazil). All other chemicals were purchased from commercial sources and were of analytical grade. Bentonite samples, characterized by Nones et al. [28,29], were collected in the city of Criciúma, located in the State of Santa Catarina, Brazil. The bentonite clays (natural or treated) were kept in a stock solution diluted in DMSO at -20°C.
Preparation of organobentonites
Organobentonite (BAC302) was synthesized by Nones et al. [17] according to the following process: 5g of bentonite was first added to 50mL of distilled water at 30°C and stirred until they were thoroughly dispersed. Desired amounts of benzalkonium chloride were mixed in 50mL distilled water at 30°C for 30min. Then the modifying agents were added to the bentonite suspension under vigorous stirring. The mixed suspensions were stirred at 30°C for 4h and then stored at room temperature (around 25°C) overnight. After that, the resulting products were washed with distilled water and dried at 80° C. The added amounts of BAC were 2% of the bentonite’s weight.
Bentonite characterization
Surface morphology of bentonites was determined by scanning electron microscope (SEM) JEOLJSM- 390LV [17]. The textural properties of bentonites were determined by Brunauer-Emmett– Teller (BET) using automated Quanta chrome Instruments (Autosorb-1). For this purpose, the samples were out-gassed at 250°C for 12h under nitrogen prior to adsorption measurement with the multi-point method. Pore distributions and pore volume were calculated using the adsorption branch of the N2 isotherms based on the Barrett–Joyner–Halenda (BJH) pore size analyzer.
Antimicrobial activity
Disc diffusion method: The modified disc diffusion method was performed according to the recommendation of the methodology of the Clinical and Laboratory Standards Institute (CLSI), approved standards M02-A10 [30]. The inoculum for the agar dilution method was prepared with growing Pseudomonas aeruginosa (ATCC 27853) to the turbidity standards. Therefore, five well-isolated colonies of the same morphology type were selected from an agar Mueller Hinton (MH) plate culture grown by 24 to 48h and transfer into a tube containing 5mL of tryptic soy broth. The culture was incubated at 35°C by 4h and adjusted until it reached the turbidity standard measured by absorbance at 625nm from 0.08 to 0.13 (1 x 108 CFU/ mL) for Pseudomonas aeruginosa, equivalent to a 0.5 MacFarland standard. Within 15 min after adjusting the turbidity of the inoculum suspension, a sterile cotton swab was dipped into the suspension and spread on the entire sterile agar MH surface. The medium surface was allowed to dry and the natural bentonite and BAC302 was deposited on top of the dry surface.
All plates were incubated at 37°C for 24h. After incubation, the presence zone of growth inhibition around the antimicrobial materials samples was observed and its diameter in millimeters was measured.
Agar dilution method: The agar dilution method was performed according to the recommendation of CLSI, approved standards M07-A8 [31]. The natural bentonite and BAC302 (in powder form) were incorporated into 25 mL of MH agar medium at 45° C and poured in Petri dishes in triplicates to achieve different concentrations (0.3, 0.6, 0.9, 1.2 and 1.5 mg/mL). The medium was mixed thoroughly with the materials and allowed to solidify at room temperature. A growth control containing only medium without the materials was performed and another growth control containing the maximum percentage of BAC incorporated in natural bentonite was performed (data not shown).
The inoculum standards were prepared similarly to the method mentioned above to obtain the concentration of 108 CFU/mL for Pseudomonas aeruginosa. The adjusted culture was diluted in 1:10 in sterile saline to obtain a concentration of 107 CFU/mL and after that 2μL drop was applied to the agar surface (~104 CFU per spot). Finally, the inoculated plates were maintained at room temperature until the moisture in the inoculum spots had been absorbed, inverted and then incubated at 35 ± 2° C for 16 to 20h. The Minimum Inhibition Concentration (MIC) was recorded as the lowest concentration of the antimicrobial material that completely inhibits bacterial growth.
Macrodilution broth method: The macrodilution broth method was performed according to the recommendation of CLSI, approved standards M07-A8 [31]. The natural bentonite and BAC302 (in powder form) were incorporated into tubes containing 2.7mL of Brain Heart Infusion (BHI) medium broth to achieve different concentrations (0.3, 0.6, 0.9, 1.2 and 1.5 mg/mL). A growth control containing only medium broth without the materials was performed. Indeed, another growth control containing the maximum percentage of BAC incorporated in natural bentonite was also realized (data not shown). The inoculum standards were prepared similarly to the method mentioned above to obtain a bacterial suspension with 108 CFU/mL concentrations. The adjusted culture was diluted in sterile broth and added in the tube containing BHI at the concentration of 105 CFU/mL. The macrodilution tubes were incubated at 35° C ± 2° C for 16 to 20h under stirring. Thereafter, an aliquot (10μL) was taken and applied on the MH agar medium and then incubated at 35 ± 2°C for 16 to 20h. Finally, the number of Colony-Forming Units (CFUs) was determined and the Minimum Bactericidal Concentration (MBC) was defined as the minimal concentrations of compounds required to kill the organisms.
Fibroblast cell cultures
Mouse embryonic fibroblast (3T3) was obtained from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle medium (DMEM-F12) supplemented with 10% of fetal bovine serum, 100 units/mL penicillin, and 100mg/mL streptomycin. 3T3 cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and routinely kept in a logarithmic growth phase through trypsinization twice a week. In addition, the culture media were changed every 2 days. For the assays, the cells were plated in 96 well plates at a seeding density of 30 thousands cells per cm2. Cell cultures (3T3) were incubated with DMSO/Benzalkonium chloride (control group), 0.3-1.5 mg/mL of natural bentonite or BAC302. After 48 h of treatment, cell viability by trypan blue or cell count analysis were determined by DAPI staining. The final concentration of DMSO in the cell culture was a maximum of 1%.
Trypan blue assay
The viability of fibroblast (3T3) cells were determined by trypan blue as described by Youn et al. [32] and Nones et al. [17,28,29]. After exposure, the cells were collected by trysination, and stained with 0.4% trypan blue solution at room temperature for 3min. The cells were then counted using a hemocytometer and a light microscope. At least one thousand cells were found and the percentages of unstained (viable) and stained (nonviable) cells were determined.
Cell count analyses
Morphology and viability of 3T3 fibroblasts were also analyzed by DAPI nuclear binding dye and optical and fluorescence microscopy (Olympus IX71). The cells were harvested and fixed with 4% paraformaldehyde for 15min without temperature control. After washing in PBS, cell nuclei were stained with DAPI and visualized and counted. At least ten fields were measured per well.
Quantitative and statistical analysis
Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test or two-way analysis of variance (ANOVA) followed by Bonferroni post-tests, using the GraphPad Prism 5.0 software. P < 0.05 was considered statistically significant. The experiments were performed in triplicate and each result represents the mean of at least three independent experiments.
Results
Morphology and chemical composition does not change after treatment with BAC
By using Scanning Electron Microscopy (SEM), we found that benzalkonium chloride did not change bentonite morphology (Figure 1A, 1B). Both samples (natural and BAC302) show individual particles, most of which had clearly recognizable contours that tended to form thick and large agglomerates. These data are in agreement with our previously results, whereby we could not see differences in natural or organic bentonites using SEM [17].
Figure 1: Morphology and chemical composition does not change after
treatment with BAC. Bentonite particles were analyzed by scanning electron
microscopy (A,B) and energy dispersive X-ray spectroscopy (C,D). Scale bar
= 100μm.
Organic treatment reduces the specific surface area and increases pore diameter of BAC302
In order to determine textural parameters of bentonite samples (natural and BAC302), the nitrogen isotherms (adsorption/ desorption) have been applied to calculate the specific surface area and pore volume, using the multipoint BET and BJH method, respectively [33].
After the organo-treatment, the specific surface area was reduced from 44.32 to 26.93 m2/g, while pore diameter was found to increase from 5.73 to 8.68 nm (Table 1). On the other hand, the pore volume of the bentonite samples slightly decreased.
Bentonite
Natural
BAC302
Specific surface area (m2g-1)
44.32
26.93
Average pore diameter (nm)
5.73
8.68
Pore volume (cm3g-1)
0.063
0.058
Table 1: Textural parameter values of natural bentonite and BAC302.
As shown in Figure 2, the nitrogen isotherms of bentonites exhibit hysteresis loops, indicating IV type isotherm, which is characteristic of mesoporematerials.
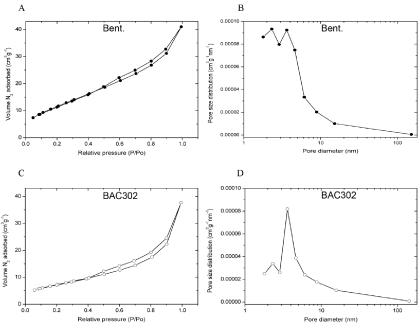
Figure 2: Organic treatment reduces the specific surface area and increases
pore diameter of BAC302. The samples were out-gassed at 250°C for 12h
under nitrogen prior to adsorption measurement with the multi-point method.
After that, nitrogen adsorption–desorption isotherms (A, B) and pore size
distributions (C, D) were determined.
The comparison of the results of organophilic bentonite (BAC302) and natural bentonite shows a decrease in the volume of adsorbed nitrogen (Figure 2A, 2C). Indeed, as shown in Figure 2B, 2D the pore size distribution of natural bentonite increased after the bentonite received the treatment.
Antimicrobial properties
The modified disc diffusion method showed an initial indication about the antimicrobial activity of BAC302. The diameter of the inhibition zone (10 ± 0.6 mm) of BAC302 indicates inhibition on growth towards Pseudomonas aeruginosa, while the natural bentonite did not show any antimicrobial effect (Figure 3).
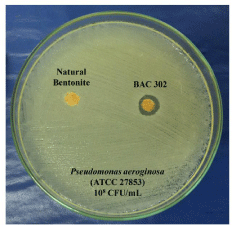
Figure 3: The new product developed (BAC302) reduces Pseudomonas
aeruginosa growth. Pseudomonas aeruginosa cultures were incubated with
natural and organo-bentonite (BAC302). By the disc diffusion method, after
24h, the antimicrobial activity of the natural bentonite (A) and BAC302 (B)
was determined.
In a second approach, the agar dilution method was applied to evaluate the antimicrobial activity of BAC302 toward Pseudomonas aeruginosa considering the values of measured colony growth diameter (in millimeters) as shown in Figure 4. Similarly to the previous test, the natural bentonite and the control group did not show antimicrobial activity, while BAC302 exhibited clear antimicrobial activity towards the bacteria in a concentration-dependent manner. At 0.6 mg/mL, BAC302 significantly reduced the bacterial growth by 46% and the MIC value was found to be at a 0.9mg/mL concentration. For this reason, higher concentrations up to 1.5mg/mL showed total reduction of bacterial growth.
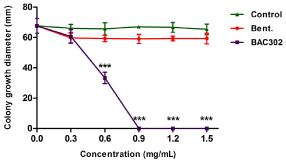
Figure 4: BAC302 has antimicrobial activity. Pseudomonas aeruginosa
cultures were incubated on agar dilution test without materials (control
group), 0.3–1.5 mg/mL of natural and organo-bentonite (BAC302). After 20h,
antimicrobial activity was analyzed. The data are shown as average values
and standard deviation of colony growth diameter in triplicates. The results
represent the mean of three independent experiments performed in triplicate
± SD. ***P < 0.01, **P < 0.1 and *P < 0.05 compared to the control or other
treatment groups.
These results agree with the one determined by the broth micro-dilution method, as shown in Table 2. None of the bentonite concentrations and the control groups reduced bacterial growth, leading to indistinct and then uncountable colony formation among the replicates. With BAC302, on the other hand, some growth inhibition was found even at the lowest concentration assayed (0.3mg/mL), since colony formation turned from indistinct and uncountable - as observed in the control group - to isolated and fairly countable (1.1x104 ± 5.0x102 CFU/mL). At 0.6 and 0.9mg/mL, CFU/ mL dropped to 3.8x103 ± 1.3x102 and 2.8x102 ± 3.5x101, respectively. Finally, there was a lack of growth inhibition at 1.2 and 1.5 mg/mL concentrations, indicating a MBC value about 1.2mg/mL.
Material Concentrations
Pseudomonas aeroginosa (CFU/mL)
Bentonite
BAC302
Control
U1
U
0.3
U
1.1x104 ± 5.0x102
0.6
U
3.8x103 ± 1.3x102
0.9
U
2.8x102 ± 3.5x101
1.22
U
-3
1.5
U
-
1Uncontable, 2MBC for BAC302, 3Absence of bacterial growth.
Table 2: Macro dilution broth method of the natural bentonite and BAC302 against Pseudomonas aeruginosa.
BAC302 at lower concentrations (0.2-0.9 mg/mL) were not found to be toxic to fibroblast
Our results indicate that both of the bentonite samples (natural or BAC302) that were tested showed an effect on Pseudomonas aeruginosa growth. In this part of the study, we have enquired if the product that was developed (BAC302) could have a toxic effect on mammal cells if applied, for example, on the skin. In order to analyze these parameters, cultures of fibroblast cells (3T3) were incubated with vehicle (control group) or 0.3–1.5 mg/mL of bentonite or BAC302 solutions. By the trypan blue assay, there was no reduction in the absorbance values after a 2-day cell treatment with 0.3 to 0.9 mg/mL when compared with the control group (Figure 5). However, a decrease in the cell viability values for the addiction of natural bentonite or BAC302 just in higher concentrations (1.2-1.5 mg/mL) (around 70 and 60% of cell viability, respectively) was found when compared to the control group.
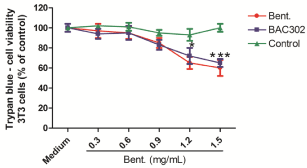
Figure 5: Natural bentonite and BAC302 at lower concentrations (0.2-0.9
mg/mL) were not found to be toxic to fibroblast. Fibroblast (3T3) and cell
cultures were incubated with DMSO (control group), 0.3–1.5 mg/mL of natural
and organo-bentonite (BAC302). After 2 days of treatment, cell viability
was analyzed by trypan blue (A). The results represent the mean of three
independent experiments performed in triplicate ± SEM. ***P < 0.01, **P < 0.1
and *P < 0.05 compared to the control or other treatment groups.
Using optical microscopy, we found that natural bentonite and BAC302 (0.6mg/mL) did not change 3T3 cell morphology (Figure 6A, 6C, 6E). Both samples (natural and BAC302) showed similar morphology when compared with the control group (Figure 6A, 6C, 6E). The same results were found by DAPI staining analysis (Figure 6B, 6D, 6F, 6G).
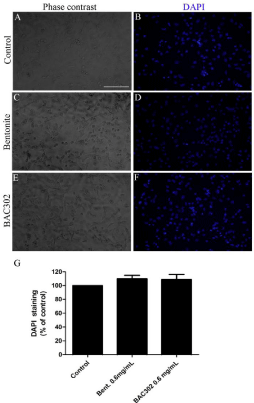
Figure 6: Natural bentonite and BAC302 does not affect morphology and
the total number for 3T3 cell population. 3T3 cell cultures were incubated
with DMSO (control group), 0.6mg/mL of bentonite or BAC302. After 48h
treatment, the total number of cell nuclei per field was determined by DAPI
(A–G). The results represent the mean of three independent experiments
performed in triplicate ± SEM. Scale bar = 50μm.
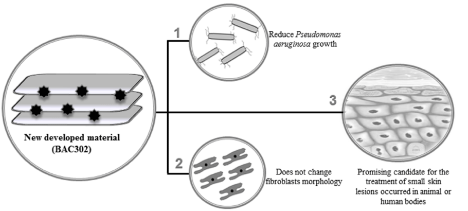
Graphical Abstract:
Discussion
The present study showed that BAC302 promotes a decrease in Pseudomonas aeruginosa growth. Moreover, our data show that these materials (natural bentonite or BAC302) are nontoxic to mammal cells (fibroblast) when used in lower concentrations.
Clay may prove valuable in the treatment of bacterial diseases, including, for example, skin infections for which there are no effective antibiotics [34]. Furthermore, different types of chemical treatment can change the properties of bentonites and montmorillonite, improving their antimicrobial action [34,35,36]. In the same way, in this study, corroborating with the findings of previous studies [20,21], we showed, for the first time, the potential effect of BAC302 (a material obtained from a Brazilian bentonite) to reduce Pseudomonas aeruginosa growth.
Although we have not found any differences in the morphology of both tested materials, the organic treatment decreased the specific surface area and increased pore diameter. The properties of bentonites are dependent on the structure and the ions present in the interlayer space and surface of bentonites [33,37]; this may possibly explain the differences between natural bentonite and BAC302 in the Pseudomonas aeruginosa growth.
The antimicrobial activity of the BAC302 proved to be important for the prevention of adherence and proliferation activities of bacteria on the surface of the materials, while natural bentonite alone did not show any antimicrobial activity. MIC and MBC were found at 0.9mg/ mL and 1.2mg/mL, respectively, but even in lower concentrations (0.6mg/mL) there was a significant reduction of bacterial growth (about ~50%).
It is known that BAC is a quaternary ammonium compound has amphiphilicity propriety (hydrophobic and hydrophilic). The hydrophilic cationic region destabilizes the pathogen surface through electrostatic interactions with negatively charged components. Thus, surface-stabilizing cations are displaced and the hydrophobic region penetrates the hydrophobic bilayer, on charged sites on bacterial cell walls, destabilizing the cell wall and the cytoplasmic membrane, which leads to cell lysis, leakage, and death [38,39,40].
The antibacterial activity of the new produced material (BAC302) is probably caused by its ability to alter the permeability of the cellular membranes, disrupting essential processes such as ATP synthesis or solute uptake; its amphiphilicity is essential in the interaction with target membranes for efficacious antimicrobial action [2,40]. The effect of BAC302 must be the result of adding BAC to bentonite, which presents significant results against the opportunist pathogen Pseudomonas aeruginosa.
Surface-active agents (surfactants) such as QACs have two regions in their molecular structures: one is a hydrocarbon, water-repellent (hydrophobic) chain and the other is a water-attracting (hydrophilic or polar) group [22]. Because of this fact, surfactants are applied as biocides which are highly efficient against lipophilic viruses, bacteria or fungi at very low concentrations [41]. This evidence suggests that organic salt (benzalkonium) interacted with bentonite by occupying the interlayer spaces, which might have contributed to maximizing the effects that were observed in the new produced material (BAC302). Our data show that BAC302 can be used effectively to control Pseudomonas aeruginosa even in lower concentrations.
It has been previously demonstrated that neuroblastoma cells briefly exposed to up to 1.0mg/mL of bentonite had their resting potentials depolarized [42]. Indeed, Elmore [43] reports that different varieties of clay minerals, including montmorillonite, are cytotoxic to macrophage cell lines and displayed hemolytic activity. Murphy et. al [44] showed that different concentrations of bentonite induce strong neurotoxic and cause a rapid degeneration of murine spinal cord neurons in the culture. However, our previous results have shown that cultures of quail Neural Crest (NC) cells showed no reduction in cell viability after a 4-day cell treatment (natural bentonite) when compared with the control group when used in lower concentrations [28]. Similarly, corroborating our previously results, our data have shown that the two materials were not found to be toxic to these cells (3T3 cells – fibroblasts) at lower concentrations (0.3-0.9 mg/mL). However, higher concentrations (1.2-1.5 mg/mL) caused a reduction on fibroblast cell viability. These findings provide information about the potential toxicity and anti-microbial attributes of natural bentonites and BAC302, thus contributing to their sustainable development as products within the safety limits.
Considering that natural bentonite can also have a protective effect, e.g., decreasing inflammation, improving the skin barrier function and acting physically through skin protection [16,45,46], our findings provide critical information about the potential bactericide effect promoted by BAC302, which may contribute to their sustainable development as products in order to protect small inflammatory responses or small cuts in animal or human skin.
Our results show that the new material that was developed (BAC302) promotes a decrease in Pseudomonas aeruginosa growth. Moreover, our data show that natural bentonite and BAC302 are nontoxic to mammal cells (fibroblast) when used in lower concentrations. The results indicate that BAC302 may represent a novel strategy to prevent Pseudomonas aeruginosa-caused diseases; it is a specially promising candidate for the treatment of small skin lesions occurred in animal or human bodies.
Acknowledgements
We would like to thank LCME-UFSC. This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico – (CNPq, MCTI, Brazil– grant number 403244/2015-3). Fundação de Amparo à Pesquisa do Estado de Santa Catarina (FAPESC, SC, Brazil).
References
- Gupta AK, Shashi S, Mohan M, Lamba IM. Epidemiology of Pseudomonas aeruginosa infections in a neonatal intensive care unit. J Trop Pediatr 1993; 39: 32-36.
- Özdemir G, Limoncu MH, Yapar S. The antibacterial effect of heavy metal and cetylpridinium-exchanged montmorillonites. Appl Clay Sci 2010; 48: 319- 323.
- Opperman MJ, Shachar-Hill Y. Metabolic flux analyses of Pseudomonas aeruginosa cystic fibrosis isolates. Metab Eng 2016; 38: 251-263.
- Gras-Le Guen C, Lepelletier D, Debillon T, Gournay V. Contamination of a milk bank pasteuriser causing a Pseudomonas aeruginosa outbreak in a neonatal Intensive care unit. Arch Dis Child Fetal Neonatal. 2003; 88: 434- 435.
- Sánchez-Carrillo C, Padilla B, Marín M, Rivera M. Contaminated feeding bottles: the source of an outbreak of Pseudomonas aeruginosa infections in a neonatal intensive care unit. Am J Infect Control. 2009; 37: 150-154.
- Garg S, Shakya N, Srivastav NC, Agrawal B. Investigation of C- 5 alkynyl (alkynyloxy or hydroxymethyl) and/or N 3 propynyl substituted pyrimidine nucleoside analogs as a new class of antimicrobial agents. Bioorg Med Chem. 2016; 24: 5521-5533.
- Machado I, Coquet L, Jouenne T, Pereira MO. Proteomic approach to Pseudomonas aeruginosa adaptive resistance to benzalkonium chloride. J Proteomics. 2013; 89: 273-279.
- Poole K. Stress responses as determinants of antimicrobial resistance in Pseudomonas aeruginosa: multidrug efflux and more. Can J Microbiol. 2014; 60: 783-791.
- Murugan N, Malathi J, Umashankar V, Madhavan HN. Unraveling genomic and phenotypic nature of multidrug-resistant (MDR) Pseudomonas aeruginosa VRFPA04 isolated from keratitis patient. Microbiol Res. 2016; 193: 140-149.
- Rybak MJ, Akins RL. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs. 2001; 61: 1-7.
- Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2011; 2: 1-13.
- Aguzzi C, Cerezo P, Viseras C, Caramella C. Use of clays as drug delivery systems: possibilities and limitations. Appl Clay Sci. 2007; 36: 22-36.
- Viseras C, Cerezo P, Sanchez R, Salcedo I. Current challenges in clay minerals for drug delivery. Appl Clay Sci. 2010; 48: 291-295.
- Dário GM, Silva GG, Gonçalves DL, Silveira P. Evaluation of the healing activity of therapeutic clay in rat skin wounds. Mater Sci Eng C Mater Biol Appl. 2014; 43: 109-116.
- Abbès S, Salah-Abbès JB, Hetta MM, Ibrahim M. Efficacy of Tunisian montmorillonite for in vitro aflatoxin binding and in vivo amelioration of physiological alterations. Appl Clay Sci 2008; 42: 151-157.
- Nones J, Riella HG, Trentin AG, Nones J. Effects of bentonite on different cell types: a brief review. Appl Clay Sci. 2015; 105-110: 225-230.
- Nones J, Nones J, Poli A, Trentin A. Organophilic treatments of bentonite increase the adsorption of aflatoxin b1 and protects stem cells against cellular damage. Colloid Surface B. 2016; 145: 555-561.
- Kevadiya BD, Chettiar SS, Rajkumar S, Bajaj HC. Evaluation of clay/ poly (L-lactide) micro composites as anticancerdrug, 6-mercaptopurine reservoir through in vitro cytotoxicity, oxidative stress markers and in vivo pharmacokinetics. Colloid Surface B. 2013; 112: 400-407.
- Modabberi S, Namayandeh A, López-Galindo A, Viseras C. Characterization of Iranian bentonites to be used as pharmaceutical materials. Appl Clay Sci. 2015; 116-117: 193-201.
- Rawat K, Agarwal S, Tyagi A, Verma AK. Aspect ratio dependent cytotoxicity and antimicrobial properties of nanoclay. Appl Biochem Biotechnol 2014; 174: 936-944.
- Brennan FP, Moynihan E, Griffiths BS, Hillier S. Clay mineral type effect on bacterial enteropathogen survival in soil. Sci Total Environ. 2014; 468-469: 302-305.
- Sütterlin H, Alexy R, Kümmerer K. The toxicity of the quaternary ammonium compound benzalkonium chloride alone and in mixtures with other anionic compounds to bacteria in test systems with Vibrio fischeri and Pseudomonas putida. Ecotoxicol Environ Saf. 2008; 71: 498-505.
- Antunes SC, Nunes B, Rodrigue S, Nunes R, Fernandes J, Correia AT. Effects of chronic exposure to benzalkonium chloride in Oncorhynchus mykiss: cholinergic neurotoxicity, oxidative stress, peroxidative damage and genotoxicity. Environ Toxicol Pharmacol. 2016; 45: 115-122.
- Lavorgna M, Russo C, D’Abrosca B, Parrella A. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magnaand Ceriodaphnia dubia as model systems. Environ Pollut. 2016; 210: 34-39.
- Pernak J, Mirska I, Kmiecik R. Antimicrobial activities of new analogues of benzalkonium chloride. Eur J Med Chem. 1999; 34: 765-771.
- Kawabata M, Ohori J, Kurono Y. Effects of benzalkonium chloride on histamine H1 receptor mRNA expression in nasal epithelial cells. Auris Nasus Larynx 2016; 43: 685-688.
- Maisanaba S, Hercog K, Filipic M, Jos Á. Genotoxic potential of montmorillonite clay mineral and alteration in the expression of genes involved in toxicity mechanisms in the human hepatoma cell line HepG2. J Hazard Mater. 2016; 304: 425-433.
- Nones J, Nones J, Riella HG, Kuhnen NC. Bentonite protects neural crest stem cells from death caused by aflatoxin B1. Appl Clay Sci. 2015; 104: 119- 127.
- Nones J, Nones J, Riella HG, Poli A. Thermal treatment of bentonite reduces aflatoxin b1 adsorption and affects stem cell death. Mater Sci Eng C Mater Biol Appl. 2015; 55: 530-537.
- CLSI. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. Wayne, Pennsylvania, USA. 2009.
- CLSI. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically. Wayne, Pennsylvania, USA. 2009.
- Youn H, Jeong JC, Jeong YS, Kim EJ. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol Pharm Bull 2013; 36: 944-951.
- Nones J, Nones J, Riella HG, Poli A. Calcination of brazilian bentonite affects its structural properties and reduces ability to bind afb1. Int J Appl Res Technol. 2016; 5: 29-36.
- Santos MF, Oliveira CM, Tachinski CT, Fernandes MP. Bactericidal properties of bentonite treated with Ag+ and acid. Int J Miner Process. 2011; 100: 51-53.
- Phukan A, Bhattacharjee RP, Dutta DK. Stabilization of SnO2 nanoparticles into the nanopores of modified montmorillonite and their antibacterial activity. Adv Powder Technol. 2016; 28: 139-145.
- Martucci JF, Ruseckaite RA. Antibacterial activity of gelatin/copper (II)- exchanged montmorillonite films. Food Hydrocolloids. 2017; 64: 70-77.
- Hanuláková D, Zeman J, Vašícek R, Prikryl R. Determination of pore water composition during long term interaction of bentonite substrates with water media: comparative study. Appl Clay Sci. 2013; 80-81: 69-75.
- azlara A, Ekhtelat M. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. American-Eurasian J Agric & Environ Sci. 2012; 12: 23-29.
- Mangalappalli-Illathu AK, Korber DR. Adaptive resistance and differential protein expression on Salmonella enterica serovar enteritidis biofilms exposed to benzalkonium chloride. Antimicrob Agents Chemother. 2006; 50: 3588-3596.
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999; 12: 147-179.
- Koziróg A, Brycki B. Monomeric and gemini surfactants as antimicrobial agents - influence on environmental and reference strains. Acta Biochim Pol. 2015; 62: 879-883.
- Banin E, Meiri H. Toxic effects of alumino-silicates on nerve cells. Neuroscience. 1990; 39: 171-178.
- Elmore AR. Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium silicate, zirconium silicate, attapulgite, bentonite, Fuller’s earth, hectorite, kaolin, lithium magnesium silicate, lithium magnesium sodium silicate, montmorillonite, pyrophyllite, and zeolite. Int J Toxicol. 2003; 22: 37-102.
- Murphy EJ, Roberts E, Anderson DK, Horrocks LA. Cytotoxicity of aluminum silicates in primary neuronal cultures. Neuroscience. 1993; 57: 483-490.
- Emami-Razavi SH, Esmaeili N, Forouzannia SK, Amanpour S. Effect of bentonite on skin wound healing: experimental study in the rat model. Acta Med Iran. 2006; 44: 235-240.
- Cervini-Silva J, Camacho AN, Kaufhold S, Ufer K. The anti-inflammatory activity of bentonites. Appl Clay Sci. 2015; 118: 56-60.