
Research Article
J Bacteriol Mycol. 2017; 4(3): 1052.
Analysis of Intestinal Bacterial Communities in Four Group Abnormal Hilit Hyperpietics and Normal Hilit of Uyghur Nationality
Yimiti D¹, Abudoukerimu A²*, Jiangwei², Tieliewuhan N³ and Wufuer H4
¹Scientific Research Exploitation Office, Xinjiang Medical University, China
²Department of Microbiology, Xinjiang Medical University, China
³Ultrasound Department, Xinjiang Medical University Affiliated First Hospital, China
4Traditional Uighur Medicine Institute, Xinjiang Medical University, China
*Corresponding author: Azierguli Abudoukerimu, Department of Microbiology, Xinjiang Medical University, China
Received: May 17, 2017; Accepted: June 12, 2017; Published: June 19, 2017
Abstract
Objective: The microbial diversity off our abnormal hilit hyperpietics and normal hilit group was investigated using nested PCR–Denaturing Gradient Gel Electrophoresis (DGGE).
Methods: The total DNA in the fecal specimens of abnormal Sawda hilit hyperpietics with (20 samples) and normal Sawda hilit healthy people (20 samples), abnormal Khan hilit hyperpietics (20 samples) and normal Khan healthy people (20 samples), abnormal Sapra hilit hyperpietics (10 samples) and normal Sapra hilit healthy people (10 samples), abnormal Balgham hilit hyperpietics with (10 samples) and normal Balgham hilit healthy people (10 samples) were used as templates. After, the 16SrDNA genes (V6-V8 region) were amplified by using the universal primers and analyzed by DGGE. On each band on gel electrophoresis, use Quantity One 4.4.0 software and SPSS 17.0 analysis software for data analysis.
Results: (1) The results from UPGAMA cluster showed the similarity of total intestinal tract bacteria. Abnormal Sawda hilit hyperpietics (45%- 87%) > abnormal Khan hilit hyperpietics (50-85%) > abnormal Balgham hilit hyperpietics (42%-86%) > abnormal Sapra hilit hyperpietics (54-82%), normal Sawda hilit (40%-80%) > normal Khan hilit (49%-79%) > normal Balgham hilit (43%-72%) > normal Sapra hilit (52%-75%) (2). By analyzing the diversity, richness and evenness of four groups of total intestinal bacteria, abnormal Khan hilit hyperpietics and normal Khan hilit, abnormal Balgham hilit hyperpietics and normal Balgham hilit was statistically significant (P< 0.05). Abnormal Sawda hilit hyperpietics respectively compared with abnormal Khan hilit hyperpietics, abnormal Balgham hilit hyperpietics was statistically significant (P<0.05). Compared with abnormal Sapra hilit, the richness and diversity of total intestinal bacteria was statistically significant (P<0.05), but the evenness was not significant (P>0.05). (3) The sequences that frequency much can representative each sample were compared with sequences in the Gene bank nucleotide sequence database by using Blast software and phylogenetic trees were constructed. Conclusion: (1) There were some differences of similarity, species and distribution diversity in intestinal microbial communities of four abnormal hilit hyperpietics and normal hilit group (2). The phylogenetic trees of intestinal flora diversity of four abnormal hilit hyperpietics and normal hilit group in Uighur nationality and the phylogenetic trees show the existence of Firmicutes, Bacteroidetes, Verrucomicrobia, Butyrate-producing bacterium and Uncultured bacterium were some factors that can be determinant for the form and development of abnormal hilit hypertension.
Keywords: Uyghur medicine; Abnormal hilit hyperpietics; Normal hilit; Intestinal microbial; Diversity; 16SrDNA-PCR-DGGE
Introduction
Uygur medicine theory considers that humans have four kinds of body fluids, including Sapra hilit, Kan hilit, Belghem hilit and Savda hilit. These four kinds of fluids mutual conversion in the body, supplement and continuously circulate throughout the body the body’s activities maintain a certain percentage balance in the whole life action. If the above four kinds of body fluids have qualitative change in proportion, it will produce abnormal hilit that is harmful to human body fluids. Uyghur medicine thinks that body fluid anomaly change is the fundamental of disease production. According to the degree of change, the role of play and the resulting symptoms and cause by disease type, etc., it will be divided into four species of abnormal body fluid: abnormal Saprahilit, abnormal Kanhilit, abnormal Belghemhilit and abnormal Savda hilit, especially the increase of abnormal Savda is the main character of abnormal changes in body fluids [1], and other abnormal body fluids through long-term retention and “burning” will produce abnormal Savda hilit, which is one of the main reason abnormal disease-causing body fluids [2-3], it will cause more serious consequences, it is the common pathophysiological basis of the tumor, hypertension, asthma, diabetes and other complex diseasein the process of development [4].
Essential Hypertension (EH) is one of the common heart cerebrovascular diseases. In recent years, its prevalence is rising worldwide. According to the epidemiological survey [5], the prevalence of essential hypertension in China continued to increase and showed a trend of younger age. Essential Hypertension has high morbidity, low control rate, more complications, it brings a heavy burden to individuals, families and society, and it has become a control key in chronic non-communicable diseases. Xinjiang is a multi-ethnic region, because of its unique geographical location, special diets and ethnic distribution. It shows a unique in the prevalence of many diseases. Epidemiological studies show that ethnic minorities in Xinjiang-China’s Xinjiang Uygur region, the prevalence of hypertension is very high and 35 residents over the age of the prevalence of hypertension have a rising tendency [6].
The human being is “the super organism” composed by 10% human body and 90% microorganism cells [7]. The human endogenous gut microflora is an immensely diverse ecosystem with approximately 1014, 1000 kinds of microorganisms inhabiting there. Gastro-intestine was divided into Physiological symbiosis with the host bacteria (eg. Baeteroidaeeae, Bifidobaceterium, Bacteroidetes, etc.), and the conditions of the host symbiotic bacteria, based mainly on facultative anaerobes (eg. intestinal coil enterococci etc.) and the majority passing bacteria Pathogens (eg. Proteus, Vibrio cholerae, Shigella, etc.). The intestinal tract bacteria are mainly in the colon area, all of which are maintaining symbiotic and antagonistic relationship. In digestion, nutrient absorption, energy supply, fat metabolism, immune regulation, drug metabolism toxicity, and many other aspects have an effect on human and animal’s health [8-10].
In recent years, with the rapid development of molecular biology, molecular biology techniques in micro-ecology are increasingly widespread, which is characterized by microbial population quickly obtaining qualitative and quantitative data. It makes micro-ecology of the existing research range be further expanded. The 16SrRNA genebased denaturing gradient gel electrophoresis (Denaturing Gradient Gel Electrophoresis, DGGE) techniques quickly and accurately identify the natural environment or human environment, microbial population, and conduct the complex microorganism group structure succession rule research, as well as biological Population’s dynamic analysis [11]. Especially in the last few years, domestic and foreign scholars use different molecular biology methods to conduct research on baeterium16S rRNA, which already obtained widespread 16SrRNA sequence database, and made the foundation for us to compare the micro ecology research. This technique allows the visualization of the structure of bacterial communities in multiple clinical specimens at a time, including culture-difficult or as-yet-uncultivated taxa in the fingerprints [12]. The present study was undertaken to compare the bacterial communities present in abnormal hilit hyperpietics group and normal hilit healthy group by using a 16SrRNA gene-based broad-range PCR-DGGE approach.
Materials and Methods
Subjects
Samples were collected from Yutianxian and Chelexian in Hetian region of Xinjiang from adult people (ages ranging from 35 to 55 years), no limit to the gender. According to Uygur medical basic theory study and hypertension of WHO diagnostic criteria, all subjects were divided into four groups: abnormal Sawda hilit hyperpietics (20 samples) and normal Sawda Hilit healthy people (20 samples), abnormal Khan hilit hyperpietics (20 samples) and normal Khan healthy people (20 samples), abnormal Sapra hilit hyperpietics (10 samples) and normal Sapra Hilit healthy people (10 samples), abnormal Balgham hilit hyperpietics (10 samples) and normal Balgham Hilit healthy people (10 samples). These people were recruited in January to February 2010. At the same time, record client’s age, gender, education, smoking history and drinking habits.
Fecal specimens were collected from each people for a total of 2 specimens per people, which were then stored at -80°C prior to being analyzed. Sample taking and DNA extraction procedures were based on QIAamp DNA Stool Mini Kit (QIAGEN, Germany), according to the manufacturer’s instructions with minor modification. All DNA was stored at -20°C before further analysis.
PCR amplification
A 16SrRNA gene fragment corresponding to nucleotide positions 968–1401 was amplified from DNA extracts of clinical samples using the following universal bacterial primers: 968f (5’-AAC GCG AAG AAC CTT AC3’)containing a 40bp GC clamp (5’-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G-3’) added to its 5’end, which makes it suitable for DGGE, and 1401r (5’-CGG TGT GTA CAA GAC CC-3’) [13]. The GC-clamp, which is a sequence that is rich in guanine and cytosine, is added to the 5’ end of the forward or reverse primer in order to prevent DNA from being completely denatured into single strands. Subsequently, this improves band resolution in denaturing gels.
The final PCR reaction mixture (50μl total volume) contained 25μl (2×PCR Taq Mix), 20mM PCR universal primer and 6μl DNA template(approximately 10 ng/μl) and sterile ultrapure water to a final volume of 50ml. Negative controls consisting of sterile ultrapure water instead of sample were included with each batch of samples analysed. PCR amplification was performed in a DNA thermocycler. The temperature profile included an initial denaturation step at 94°C for 3min, followed by 35 cycles of a denaturation step at 94°C for 1min, a primer annealing step at 62.5°C for 1min, an extension step at 72°C for 1min and a final step of 72°C for 10min. Before the DGGE analysis, the presence of PCR products was checked by electrophoresis in a 2% agarose gel stained with ethidium bromide in 1×TAE buffer using 2000bp DNA ladder digestas the molecular size standard. The gel was stained for 15min with 0.5μg GoldView ml- 1and viewed under UV transilluminator and photographed.
DGGE assay
DGGE of PCR products generated with the 968f-GC/1401r primer set was performed using the Dcode TM Universal Mutation Detection System (Bio-Rad). Equal masses of PCR products were separated on 6% (wt/vol) polyacrylamide gels (40% acrylamide/ bis solution, 37.5:1) in 1×TAE buffer (40mM Tris, 20mM Acetate, 1.0Mm Na2EDTA) using denaturing gradient ranges of 20 % to 70 % denaturant (100% denaturing solution contains 7mol urea-1and 40% (v/v) deionized form amide). Electrophoresis was performed at 50V for 30min, then at 200V for 300min. Electrophoresis buffer (1×TAE) was maintained throughout at 60°C. Gels were then stained using SYBR Green I nucleic acid stain, visualized on a UV transilluminator, and photographed.
DGGE analysis
Each DGGE experiment was repeated at least twice to ascertain the reproducibility of the bands. In this study, the diversity, richness and evenness of intestinal flora were analyzed and the similarity of UPGMA cluster analysis results were compared to reflect the features of intestinal flora of these commonly used four groups DNA extraction kits and to further provide some basic data for intestinal microflora study. Richness (S): number of bands in each lane; the Shannon-Wiener index of microflora (H):H=-S (Pi) (Ln Pi), Pi: the relative abundance of each species, calculated as the proportion of individuals of a given species to the total number of individuals in the community; the Evenness (E): E=H/H’ max, H’max=Ln S. Individual lanes of the DGGE gel images were straightened and aligned with Quantity One 4.4.0 software. The number of bands in normal hilithealthy group and abnormal hilit hyperpietics group was compared by using Student’s t-test of SPSS 17.0 analysis software. The prevalence of the most dominant bands was also recorded.
Results
All samples yielded an amplified one of the expected size after 16SrRNA gene-based broad-range PCR, indicating the presence of bacterial DNA. Figure 1 depicts cluster analysis of profiles obtained from DGGE banding patterns for abnormal hilit hyperpietics group and normal hilit healthy group. There was a largeinter-individual variability.
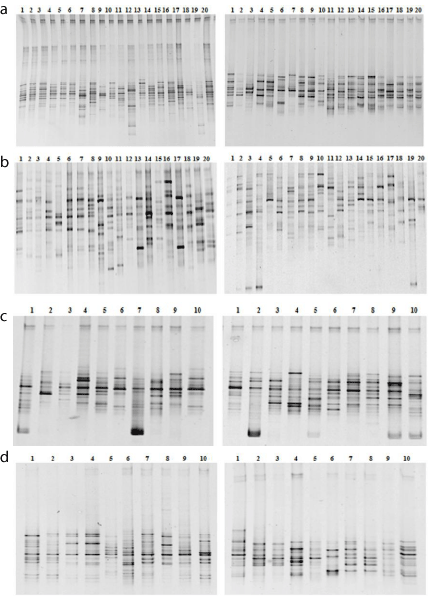
Figure 1: DGGE profiles of intestinal bacterial communities 16S rRNA gene
amplified from abnormal Sawda hilit and normal Sawda hilit (a), abnormal
Khan Hilit and normal Khan hilit (b), abnormal Sapra hilit and normal Sapra
hilit (c), abnormal Balgham hilit and normal Balgham hilit samples (d) (leftabnormal,
right- normal). A 20–70% denaturing gradient was used.
By analyzing the diversity, richness and evenness of four groups of total intestinal bacteria (Table 1), the difference of richness between abnormal Sawda hilit hyperpietics and normal Sawda hilit was statistically significant (P<0.05), but no significant differences of diversity and evenness were found (P>0.05). The difference of diversity, richness and evenness between abnormal Khan hilit hyperpietics and normal Khan hilit was statistically significant (P<0.05). The difference of diversity and richness between abnormal Sapra hilit hyperpietics and normal Sapra hilit was statistically significant (P<0.05), but no significant differences of evenness were found (P> 0.05). The difference of diversity, richness and evenness between abnormal Balgham hilit hyperpietics and normal Balgham hilit was statistically significant (P<0.05). The difference of diversity, richness and evenness between abnormal Sawda hilit hyperpietics and abnormal Khan hilit hyperpietics, abnormal Balgham hilit hyperpietics was statistically significant (P<0.05), the difference of diversity, richness between abnormal Sawda hilit hyperpietics and abnormal Sapra hilit hyperpietics was statistically significant (P<0.05), but no significant differences of evenness were found (P>0.05).
Group
Richness
H
Evenness
Abnormal Sawda hilit
8.250±1.585*
2.065±O.201
0.987±0.008
Normal Sawda hilit
9.200±2.628*
2.145±0.278
0.983±0.009
Abnormal Khan Hilit
13.300±2.452*#
2.518±0.186*#
0.979±0.009*#
Normal Khan Hilit
8.200±1.473*
2.024±0.182*
0.967±0.014*
Abnormal Sapra hilit
7.700±1.567*
1.965±1.978*
0.972±0.010#
Normal Sapra hilit
10.300±1.494*
2.236±0.144*
0.964±0.013
Abnormal Balgham hilit
6.500±1.354*#
1.734±0.232*#
0.935±0.022*#
Normal Balgham hilit
10.700±1.418*
2.295±0.124*
0.971±0.006*
Table 1: Diversity indices calculated from the DGGE banding profiles generated from V6-V8 region. (*indicate statistical significance compared with the control group (P<0.05), #indicate statistical significance compared with the abnormal Sawda hilit hyperpietics (P<0.05)).
The results from UPGAMA clustering (Figure 2) showed that the total intestinal tract bacteria between abnormal Sawda hilit hyperpietics (45%-87%) and normal Sawda hilit (40%-80%) had certain similarity, abnormal Khan hilit hyperpietics (50-85%) was more highly similar than normal Khan hilit (49%-79%), abnormal Sapra hilit hyperpietics (54-82%) was more highly similar than normal Sapra hilit (52%-75%), abnormal Balgham hilit hyperpietics (42%-86%) was more highly similar than normal Balgham hilit (43%- 72%), abnormal Sawda hilit hyperpietics was more highly similar with and abnormal Khan hilit hyperpietics, abnormal Balgham hilit hyperpietics comparing with abnormal Sapra hilit hyperpietics.
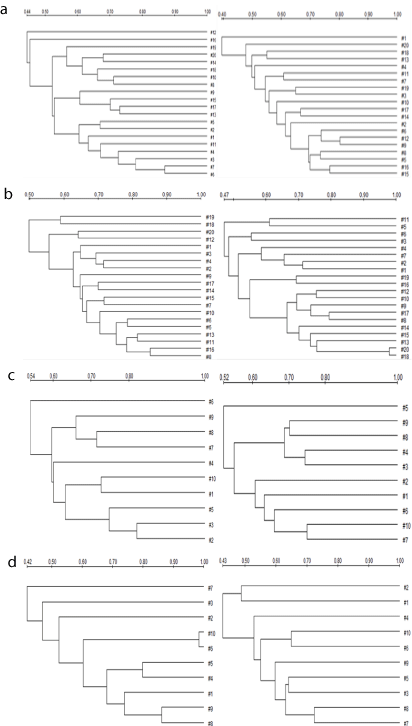
Figure 2: UPGMA cluster analysis of profiles obtained from DGGE banding
patterns for abnormal hilit hyperpietics group and normal hilit healthy group
for similarity coefficients. abnormal Sawda hilit and normal Sawda hilit (a),
abnormal Khan Hilit and normal Khan Hilit (b), abnormal Sapra hilit and
normal Sapra hilit (c), abnormal Balgham hilit and normal Balgham hilit (d)
(left-abnormal, right-normal).Scale shown is similarity of UPGMA cluster.
According to the analysis of brand markers (Figure 3), the phylogenetic trees (Figure 4) show that the existing Firmicutes, Bacteroidetes, Verrucomicrobia, Butyrate-producing bacterium and uncultured bacterium were some factors that can be determinant for the form and development of abnormal hilit hypertension.
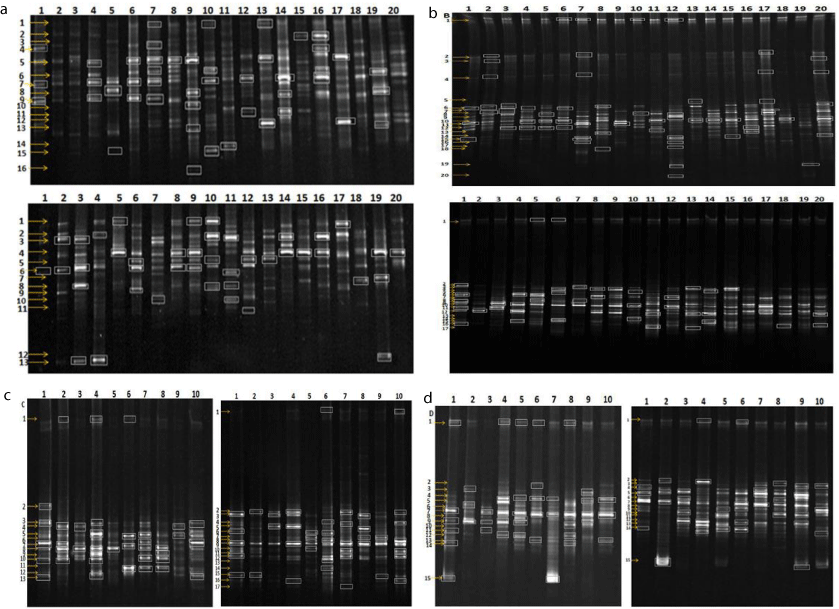
Figure 3: The markers in abnormal Sawda hilit and normal Sawda hilit (a), abnormal Khan Hilit and normal Khan hilit (b), abnormal Sapra hilit and normal Sapra
hilit (c), abnormal Balgham hilit and normal Balgham hilit samples (d) (left- abnormal, right- normal).
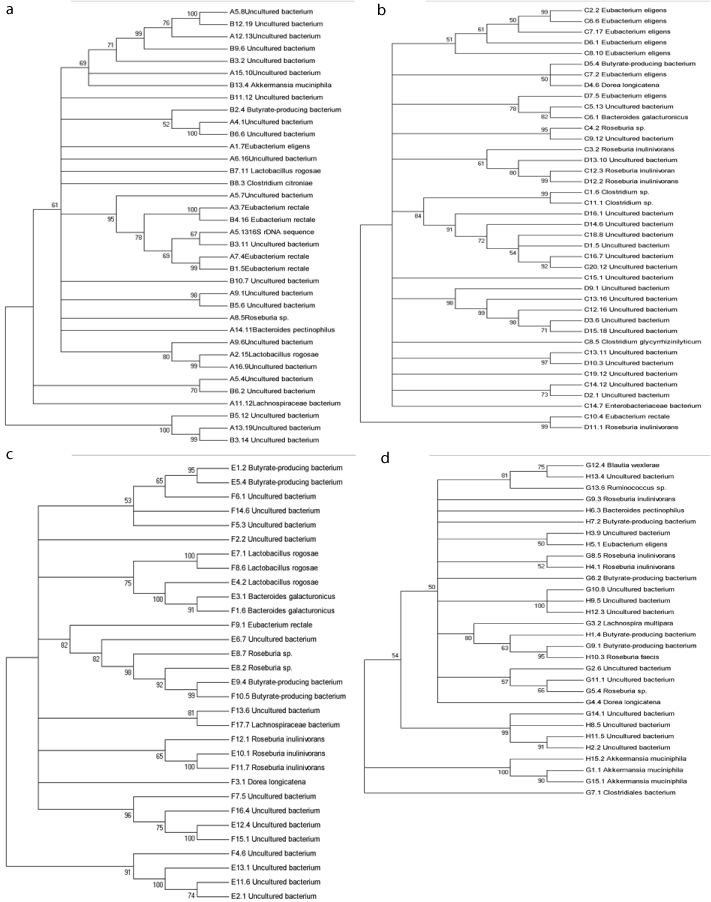
Figure 4: The phylogenetic trees of intestinal flora diversity in four abnormal hilit hyperpietics and normal hilit group in Uyghur nationality. Abnormal Sawda hilit and
normal Sawda hilit (a), abnormal Khan Hilit and normal Khan Hilit (b), abnormal Sapra hilit and normal Sapra hilit (c), abnormal Balgham hilit and normal Balgham
hilit (d).
Discussion
The rationale for bacterial species to be distinguished by the DGGE approach lies in the fact that different bacterial species present different nucleotide sequences within the variable regions of the 16SrRNA gene, making PCR amplicons migrate differently in the DGGE gel. Theoretically, each band in the polyacrylamide DGGE gel represents a certain species, although it must be recognized that there are several factors that may overestimate or underestimate the community diversity as revealed by DGGE. From this study, we are aware that the DGGE technique is an effective and convenient method for detecting intestinal bacterial communities in four type hilit. Further studies using sensitive quantitative molecular biology techniques, such as real-time PCR, are required to compare bacterial counts in abnormal hilit hyperpietics group and normal hilit healthy group and then help elucidate this issue.
Blood pressure and high-fat, high protein diet dietary pattern, low-fat, low-protein nutritional increases are related. Uygur high carbohydrate imbalance and severe shortage of large small nutrition, perhaps because of this imbalance, diet makes a high incidence of hypertension. Have reports in the literature, high protein and high fat diet to enhance the propagation of harmful bacteria intestines, resulting in promotion of vasoconstriction and rise in blood pressure of toxic substances [14]. Dwelling in the human intestinal bacteria is not only involved in a variety of host nutrient metabolism in vivo, but produces material that the body itself can’t synthesis. Some probiotics secreted proteins can inhibit the activity of the competitive ACE, thus play a role in lowering blood pressure [15]. The evidence showed that the intestinal bacteria, through the metabolic material, affects blood pressure levels.
In human body, the intestinal flora is almost following with all life. Neonatus is a sterile microenvironment, but within a few days after birth can establish the physiological microflora model, known as colonization. But always accompanied by re-colonization of people’s life, not only with diet, lifestyle, health conditions and the use of antibiotics and the like, and in different ages, and then the structure of bacteria differ in re-colonization. Epidemiological studies show that the prevalence of hypertension is very high and 35 residents over the age of the prevalence of hypertension have a rising tendency [6] in Xinjiang-China’s Xinjiang Uygur region. Therefore, this study selected 35-55 years old age groups. Uygur medicine considers that Kan hilit population had well-developed fat, while the Balgham hilit people had poor developed fat, which may one reason of intestinal microflora differences between Kan hilit and Balgham hilit. In addition, diet, endocrine, and health habits also affect the distribution difference of intestinal microflora.
This study which is based on Uyghur medicine hilit classification, analysis of people of different body fluids of the intestinal flora similar in structure and diversity, and further combined with the basic theory of Uyghur medicine hilit and modern micro-ecology, reveals the structure distribution in intestinal microflora of four abnormal hilit hyperpietics and normal hilit group and provides a theoretical basis for the theory of modern Uygur medicine and scientific applications.
References
- Kurax T, Abdurehim Y, Halmurat U. Journal of Medicine and Pharmacy of Chinese Minorities. 2004; 3: 3-4.
- Halmurat U. Therapy of Mizaj and Hilit in Uyghur Medicine and Modern Study [M]. Urumqi: Science and Technique Publishing Company in Xinjiang. 2003: 44-45.
- Abdurehim Y, Lin L, Halmurat U, et Al. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2004; 10: 61-62.
- Ablimit Y. Basic Therapy of Uyghur Medicine [M]. Urumqi: Science and Technique Publishing Company in Xinjiang. 1988: 32-46.
- Hyman DJ, Pavlik VN. Poor Hypertension Control: Let’s Stop Blaming The Patients [J]. Cleve Clin J Med. 2002; 69: 793-799
- Jianzhong L, Jianping W, Fanka L. Prevalence of Hypertension among Residents in Xinjiang Production and Construction Corps [J]. Journal of Preventive Medicine Information. 2011; 27: 120-121.
- Lederberg J. Infectius History. Science. 2000; 288: 287-293.
- Ley RE, Peterson DA, Gordon JI. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell. 2006; 124: 837-848.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the Human Intestinal Microbial Flora. Science. 2005; 308: 1635-1638.
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic Analysis of the Human Distal Gut Microbiom. Science. 2006; 312: 1355-1359.
- Gauffin Cp, Aguero G, Perdigon G. Adjuvant Effects of Lactobacillus Casei Added To Renutrition Diet in Malnourished Mouse Moder [J]. Biocell. 2002; 26: 35-48.
- Siqueira JF, Rôças IN, Rosado AS. Investigation Of Bacterial Communities Associated With Asymptomatic And Symptomatic Endodontic Infections By Denaturing Gradient Gel Electrophoresis Fingerprinting Approach. Oral Microbiol Immunol. 2004; 19: 363-370.
- Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, et al. Sequence Heterogeneities of Genes Encoding 16s Rrnas In Paenibacillus Polymyxa Detected By Temperature Gradient Gel Electrophoresis. J Bacteriol. 1996; 178: 5636-5643.
- Augenlicht Lh, Mariadason Jm, Wilson A, Arango D, Yang W, Heerdt Bg, et al. Short Chain Fatty Acids And Colon Cancer. J Nutr. 2002; 132: 3804-3808.
- Segain Jp, Raingeard De La Bletiere D, Bourreile A, Leray V, Gervois N, Rosales C, et al. Butyrate Inhibits Inflammatory Responses Through Nfkappab Inhibition: Implications For Crohn’s Disease. Gut. 2000; 47: 397- 403.