
Research Article
J Bacteriol Mycol. 2017; 4(4): 1056.
Evaluation of Antisseptic Effects on the Eye Surface and the Role of Gentamicin in Microbial Control of Donor Corneas
Ito CRM¹*, de Braga CASB², Carneiro LC², Messias ACMC², Feitosa APP¹, de Moraes AP², de Queiróz PHP2, Monteiro JC1¹ and de Ávila MP²
¹Center of Reference in Ophthalmology, Federal University of Goiás, Brazil
²Institute of Tropical Pathology and Public Health, Federal University of Goiás, Brazil
*Corresponding author: Célia Regina Malveste Ito, Center of Reference in Ophthalmology, Federal University of Goiás, Brazil
Received: October 09, 2017; Accepted: November 14, 2017; Published: November 21, 2017
Abstract
Decontamination of the surface of the donor eyeballs with antisepsis are effective, ensuring greater transplantation safety. This study evaluated the antiseptic effect in reducing the microbiota of the ocular globe of donors of corneas, with 5% povidone-iodine (right eye) and 0.05% chlorhexidine gluconate (left eye). In the action times of 5, 10 and 15 minutes, and the susceptibility profile of the microbiota isolated from gentamicin. Swabs were collected from the ocular surface before application of the solutions, after and at the time of preservation of the corneal tissue. After identification of the microbiota, an antibiogram test was performed with gentamicin. There was a reduction of 39, 5% in the total of gram-positive bacteria, and of 76, 5% in the gram-negative bacteria, and 1.7% fungi, with no statistically significant difference (p=0.183), which demonstrated that the bacterial elimination capacity of the antiseptics was similar. Both were more effective for gram negative bacteria, with a statistically significant difference (p<0.001). In the third collection, after the residual effect of the antiseptics, there was a reduction of 99.1% of all the microorganisms. In the antibiogram test, 88% of the isolated microorganisms were sensitive to gentamicin. The use of antiseptics is essential for the effective decontamination of donated corneas prior to preservation and the residual time of the antiseptics increased the decontamination power of povidone-iodine and chlorhexidine gluconate, being similar in reducing the microbiota of the ocular globe of the donor of corneas. Gentamicin contained in the cornea preservation medium complements the antisepsis of the donated tissues.
Keywords: Eye bank; Enucleation; Povidone-iodine; Chlorhexidine gluconate; Transplantation
Introduction
Donated eyes are obtained from cadavers from non-sterile environments such as domicile, public thoroughfare, hospitals and morgues [1], and rigorous screening of the medical and social history, body and eye of the donor is required. For this, preventive strategies are used, such as exclusion of donors with septicemia or endocarditis, antiseptic preparation and decontamination of the donated tissue, as well as preservation in antibiotic containing medium. Some eye bank still performs a microbiological evaluation in order to certify the absence of microbial contamination before the distribution of corneal tissue or at the time of transplantation [2].
On the ocular surface, especially in the human conjunctiva is a resident microbiota that has a fairly uniform pattern, although slight variations of certain micro-organisms occur in some parts of the world. Among the bacteria, species such as coagulase negative Staphylococcus (SCN), Streptococcus viridans group, Corynebacterium spp. and Moraxella spp [3]. Fungi contamination can also occur, with a prevalence of 3 to 28%, especially Candida albicans, Saccharomyces cerevisiae, Cryptococcus neoformans and Aspergillus flavus, among others [4].
Among the antiseptic solutions most used by eye bank or in ophthalmologic surgeries are polyvinylpyrrolidone-iodine and gluconate of chlorhexidine gluconate. Both are broad spectrum microbicides, with rapid action and depending on the concentration used, low corneal toxicity [5].
The povidone-iodine target is the cytoplasmic membrane and its action to kill the micro-organism occurs in a few seconds, since the free iodine released will oxidize and ionize the vital molecules of the cell [6]. A 5mg/mL solution, acting for two minutes, considerably reduces microbial contamination, without damage to the corneal tissue [7].
Gluconate of chlorhexidine, in turn, is a cationic bisbiguanide, which binds electrostatically to negatively charged surfaces, with specific and strong adsorption to the phosphate-containing compounds. Upon contact with the micro-organisms, gluconate of chlorhexidine damages the outer layers of the cell wall, which makes the cell permeable and allows its entry into the cytoplasmic membrane. This cause loss of low molecular weight components, such as ions of potassium [8].
After decontamination of ocular globes using the antiseptics cited, the cornea is storage in preservation media, enriched with nutrients such as glucose, amino acids, minerals and vitamins, whose purpose is to protect cells from the córnea. The medium can have too the gentamicin and streptomycin antibiotics [9,10], allowing prolonged storage. This médium is widely used by eye bank in Brazil, as well as an analogue, but without streptomycin [11].
The objective of this study was to evaluate the antiseptic effect in the reduction of ocular globe microbiota from corneal donors, prior to enucleation with 5% povidone-iodine and 0.05% Gluconate of chlorhexidine at different application times, as well as to analyze the susceptibility of the microbiota alone to gentamicin.
Methods
The research was submitted to the Ethics Committee of the Hospital das Clínicas of the Federal University of Goiás (HC / UFG), under the number CAAE: 58444316.3.0000.5078, and was approved. All had the donation term signed by a first or second-degree relative, as well as a witness.
The evaluation of the donor’s medical history was carried out by means of medical records, exams, anamnesis by the coroner and epidemiological interviews with the family. We also documented the location, time of death and time of enucleation of the eyeball. Donors with signs or suspicions of infection were excluded from the study.
Prior to face and ocular surface antisepsis, a swab soaked in 0.9% saline solution was rubbed throughout the conjunctival fornix of the right eye and immediately transferred to a tube containing Brain Heart Infusion (BHI) broth, duly identified with the data of the donor. The same procedure was then performed on the left eye.
After this first harvest, the anti-sepsis of the donor’s face and eyelids was performed, according to the PMS) of the eye bank and it is essential to change gloves. Cleaning was performed with 0.9% saline irrigation, above the eyelids, to remove any impurity. Then, with the aid of sterile gauze, the 10% topical povidone-iodine was applied, always in the same direction. To do so, the eyes were closed tightly and the procedure performed very carefully, so that there was no penetration of the antiseptic on the ocular surface.
The eyes were then opened and the ocular surface was cleaned by irrigating 10mL of 0.9% saline solution. After the excess liquid was withdrawn with sterile gauze and with a sterile glove, the antiseptics were applied to the ocular surface.
On the ocular surface of the right eye were applied 5ml of 5% povidone-iodine diluted solution and in the left eye 5ml of 0.05% dilute Gluconate of chlorhexidine solution. After 5 minutes of antiseptic application, a new cleaning with 10mL of 0.9% saline was performed. Then a swab was passed back into the conjunctival fornix and transferred to a tube containing BHI broth, duly identified with the donor data.
This procedure was also carried out in 10 and 15 minutes, with 10 samples in each group, totaling 30 donors. After the second harvest, enucleation of the ocular globes was performed, which were sent in a humid chamber to the eye bank. In the eye bank, after biomicroscopic evaluation of the eyeball and cornea, the tissue preservation phase and the last sample harvest were initiated. In laminar flow was irrigated using saline 0.9% over the entire surface of the corneal tissue. A swab was then rubbed across the corneal surface and horn-scleral flap and immediately transferred to tube containing BHI broth.
The tubes containing the samples were immediately sent to the Laboratory of Anaerobes, Phenotyping and Molecular Biology (LAFEBIM) of the Institute of Tropical Pathology and Public Health of the Federal University of Goiás, for processing. A 0.1mL aliquot of each tube was seeded on nutrient Agar, which was incubated in aerobic at 37°C for 24 hours for bacterial counting. The tubes containing the samples were also incubated under the same conditions. After the tubes were homogenized and a 0.1mL aliquot of the broth was seeded in the Mac Conkey agar medium, Saline Mannitol agar, base agar supplemented with 5% horse defibrinated blood, which were incubated under the same conditions. A 0.1mL aliquot of the broth was also seeded on Sabouraud agar and kept at room temperature for seven days.
Afterwards, morph colonial, morphotintorial characterization and biochemical tests were performed to identify the microorganisms, as well as antibiotic testing with gentamicin. The option of using gentamicin in the antibiogram test is justified by the fact that the preservation medium used in the BTOC participant of the present study only contains this antimicrobial. To compare proportions, the Chi-square test or Fisher’s exact test, when applicable, was used; to compare continuous variables, the T-test was used; and to evaluate the reduction in the number of colonies, the paired McNemar Test was used. The level of statistical significance of 5% (p <0.05) was considered for all tests.
Results
Microbiological samples were collected from 60 eyes, which corresponded to 30 donors of corneas, and 63% (19/30) were male. Donor age had a median of 54 years, with a minimum of 29 and a maximum of 78 years (Table 1). The mean temperature at the time of collection had a median of 29°C (minimum 24°C, maximum 32°C), but the lowest temperature was observed in the group of donors who received 15-minute antiseptic treatment, whose median was 25.5°C. This lower temperature had a statistically significant analysis, with p = 0.011, for a better antiseptic effect.
Variable
Total
Group
p-value
Gender
5 minutes
(n=10)
10minutes (n=10)
15minutes (n=10)
Males (n, %)
19 (63,3)
7 (70,0)
6 (60,0)
6 (60,0)
<0,999**
Females (n, %)
11 (36,7)
3 (30,0)
4 (40,0)
4 (40,0)
-
Age (in years)
Mean (Standartdeviation)
55,2 (14,5)
57,8 (13,7)
58,1 (14,6)
49,6 (14,9)
0,341*
Median (IIQ 25-75)
54 (44-68)
57,5 (44-68)
61 (51-70)
49,5 (37-56)
-
Minimum-maximum
29-78
41-78
31-76
29-78
-
Temperature(0C)
Mean (Standartdeviation)
28,0 (2,39)
28,9 (2,4)
28,8 (1,7)
26,2 (2,2)
0,011*
Median (IIQ 25-75)
29 (25,8-29,3)
28,5 (27,5-31,3)
29(28,5-30,0)
25,5 (24-29)
-
Minimum-maximum
24-32
25-32
25-31
24-29
-
Death time for sampling(in hours)
Mean (Standartdeviation)
5:15 (1:53)
6:20 (0:59)
5:40 (1:24)
3:46 (2:09)
0,004*
Median (IIQ 25-75)
05:32 (4:52 - 6:32)
6:35 (5:45 - 7:00)
5:25 (5:17 - 6:45)
4:45 (1:10 - 5:41)
-
Minimum-maximum
1:00 - 7:00
4:00 - 7:20
2:34 - 7:30
1:00 - 6:00
-
*T-test (p <0.05). **Chi-square test (p <0.05). Statistically significant values in bold.
Table 1: Epidemiological characteristics of donors of corneas, time of withdrawal between death and enucleation, and ambient temperature observed at the moment of collection of the eyeball.
The median withdrawal time between death and enucleation of the eyeballs was 5:32 minutes, with a minimum of 1:00 minute and a maximum of 7:00 minutes. When analyzing the influence of collection time on the number of isolated micro-organisms, it can be noticed a lower contamination of the ocular surface in the group of individuals with less time of withdrawal of the eyeball. Donors who had this shortest time focused on the antiseptic treatment group for 15 minutes, which influenced the potentiation of antisepsis at this time, and was statistically significant, with a value of 0.004, as shown in Table 1.
The most frequent causes were Acute Myocardial Infarction (AMI), represented by 53.4% (16/30) of the total, followed by stroke (16.7% (5/30) and 13.4% % (4/30) died due to traumatic brain injury, 6.7% (2/30) died of acute respiratory failure and another 6.7% (2/30) of pulmonary thromboembolism, finally had a group of 3% (1/30) who died of intestinal cancer.
A 100% microbial growth was observed in all samples collected prior to application of the antiseptics, which corresponds to the ocular microbiota of the donor after death. A total of 353 microorganisms were isolated from bacteria and fungi. Gram-positive bacteria predominated, 70% (247/353), followed by gram-negative bacteria, 28.3% (100/353) and fungi 1.7 % (6/353).
Among the positive bacteria, the genus Staphylococcus was the majority (81.8%, 202/247), followed by catalase negative cocci (9.7%, 24/247). As regards Gram negative bacteria, 77% (77/100) of enterobacteria and no fermenting Gram-negative rods represented 14% (14/100) of the isolated colonies.
Fungi were isolated from four donors (1.7%), and in two, C. albicans (1.13%), was identified in both eyes; in one donor, there were Aspergillus spp. and in another, Penicillium spp. It is important to note that the fungi were only present in the first collection (data not shown).
A highly significant difference of the untreated stage for the antiseptic stage was observed at the CFUs evaluation stage from first to second collect. In the right eye, which was used povidone-iodine, the p was 0.014 (SD 592.3-82.4), already in the left eye, with the use of gluconate of chlorhexidine, the p was 0.002 (DP 247.5-22.4), which proves a significant reduction of the ocular microbiota of the corpse with the use of antiseptics (Table 2).
Statistic data
UFC
12collect (n=24)
22collect(n=24)
OD
OE
OD
OE
Mean (Standartdeviation)
305,1 (592,3)
175,2 (247,5)
25,5 (82,4)
5,1 (22,4)
PC
-
0,291
-
0,250
PE
-
-
0,014
0,002
Median (IIQ 25-75)
63 (7-411)
42,5 (6-272,5)
0 (0-393)
0 (0-0)
Minimum-maximum
1-2.800
1-790
0-8,5
0-110
UFC: Colony Forming Unit; OD: Right Eye; OE: Left Eye.
Table 2: Comparison of the Colony Forming Unit (CFU) counting average between the first and second biological sample collection of the ocular surface of corneal donors.
The most of the collections, 73% (22/30), were performed in donors at the Medicalegal Death Investigation Service (MDI) and 27% (8/30) were hospitalized in Goiânia/ GO hospitals, whose hospitalization period was, on average, eight days, with minimum time of three and maximum of 20 days. When analyzing the distribution of the microbiota in relation to the origin of the donor, there was no statistically significant difference (p> 0.05), which shows that the length of stay did not influence the composition of the ocular microbiota (Table 3).
Micro-organisms
Hospital
MDIS1
p-valor
(n=8)
(n=22)
Staphylococcusspp.
8 (100,0%)
22 (100,0%)
> 0,999
Cocos catalase negativa
2 (25,0%)
8 (36,4%)
0,904
Bacillus spp.
1 (12,5%)
4 (18,2%)
> 0,999
Corineformes
3 (37,5%)
7 (31,8%)
> 0,999
Enterobactérias
5 (62,5%)
14 (63,6%)
> 0,999
BG-NF3
-
7 (31,8%)
0,513
Vibrio spp.
-
3 (13,6%)
> 0,999
Fungi
3 (37,5%)
1 (4,5%)
0,095
1- Medicolegal Death Investigation Service; 2-BG-NF: - Non fermenting Gram-negative Bacilli.
Table 3: Comparison between the types of microorganisms isolated from the ocular surface of corneal donors with respect to the origin of the donor.
Regarding the data obtained with the use of antiseptics, in the second collection, after antisepsis, there was a reduction of 39.5% (152/92) in the total Gram-positive bacteria, and 76.5% (81/19) in the Gram negative, there being no significant statistical difference (p=0.494), which shows that the bacterial elimination capacity of antiseptics was similar for both groups. It is observed that both antiseptics were more effective for the Gram-negative, with statistically significant difference (p<0.001), than for Gram-positive, with no statistically significant difference (p=0.183) (Table 4).
Isolados
1² coleta (microbiota)
2² coleta (após antisséptico)
3² coleta (antes da preservação)
Todas as coletas
Redução (%) da 1² coleta para 2² coleta
p-valor*
OD1
OE2
Total
OD
OE
Total
OD
OE
Total
OD
OE
Total
OD
OE
Total
PVP-I6
GC7
Staphylococcus spp.
63
56
119
49
31
80
2
1
3
114
88
202
22,2
44,6
32,8
0,494
Cocos catalase negativa
9
9
18
5
1
6
-
-
-
14
10
24
44,4
88,9
66,7
0,424
Bacillus spp.
4
3
7
2
2
4
-
-
-
6
5
11
50,0
33,3
42,9
>0,999
Corineformes
4
3
7
-
1
1
-
-
-
4
4
8
100,0
66,7
85,7
-
Cocos G+3
-
1
1
-
1
1
-
-
-
-
2
2
-
0,0
0,0
-
Total bactérias G+
80
72
152
56
36
92
2
1
3
138
109
247
30,0
50,0
39,5**
0,157
Enterobactérias
30
34
64
5
8
13
-
-
-
35
42
77
83,3
76,5
79,7
<0,001
BG-NF4
4
5
9
3
2
5
-
-
-
7
7
14
25,0
60,0
44,4
0,7
Bacilos curvos
5
3
8
1
-
1
-
-
-
6
3
9
80,0
100,0
87,5
-
Total bactérias G-5
39
42
81
9
10
19
-
-
-
48
52
100
76,9
76,2
76,5**
-
Total bactérias
119
114
233
65
46
111
2
1
3
186
161
347
45,4
59,6
52,4
-
Fungos
4
2
6
-
-
-
-
-
-
4
2
6
100,0
100,0
100,0
-
Total
123
116
239
65
46
111
2
1
3
190
163
353
47,2
60,3
53,6
-
1- OD: right eye; 2- EO: left eye; 3- G+: Gram positive; 4-BG-NF Gram-negative non-fermentative Bacilli, 5-G-: Gram negative; 6- PVP-I - Povidone-Iodine, 7-GC-Chlorhexidine Gluconate. * Mc Nemar Test. **p = 0.183.
Table 4: Absolute distribution of ocular surface isolated microorganisms from 30 corneal donors, by sampling steps.
In the third collection, there was a reduction of 99.1% (350/353), due to the residual action of the antiseptics between the second collection, which comprised the enucleation process and the third, preservation of the corneal tissue, which was 2:11 minutes (DP=39), with variation between 1:18 minutes and 3:35 minutes of all micro-organisms (Figure 3), except for the growth of 0.9% (3/353) Staphylococcus spp. in a donor at the time of application of 5 minutes in both antiseptics (Figure 1).
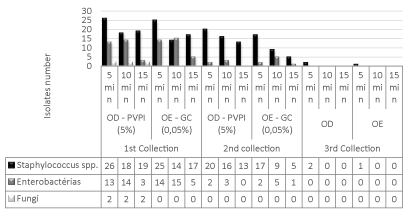
Figure 1: Comparison between microbiota reduction after application of 5%
povidone-iodine (PVP-I) antiseptics and 0.05% chlorhexidine gluconate (CG)
at 5, 10 and 15 minutes times.
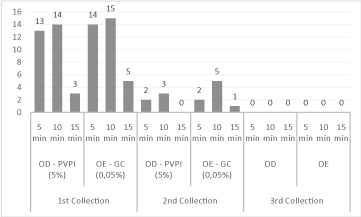
Figure 3: Reduction of the enterobacteria number between the collections,
after application of 5% povidone-iodine antiseptics (PVP-I) and 0.05%
chlorhexidine gluconate (GC), in relation to the action time.
When comparing the efficacy of the antiseptics tested, there was a reduction in the number of contaminants, both with the use of povidone-iodine and with the use of gluconate of chlorhexidine, with no significant statist difference (p>0.05) (Table 4). However, when comparing, separately, the two largest groups of isolates, Staphylococcus spp. and enterobacteria, it was possible to observe differences between the times of action of antiseptics, with reduction of contaminated donors.
For Staphylococcus spp., it was possible to observe that both povidone-iodine and gluconate of chlorhexidine were more effective with 15 minutes, with a reduction rate of 31.6%, respectively (Figure 2). As for the enterobacteria, there was a reduction of 70.6% in the action time of 5 minutes for both antiseptics (Figure 3).
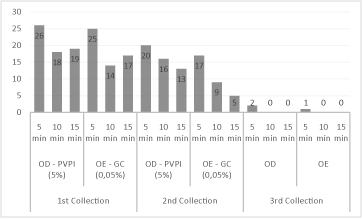
Figure 2: Reduction of Staphylococcus spp. between the collections,
after application of 5% povidone-iodine (PVP-I) antiseptics and 0.05%
chlorhexidine gluconate (GC), with respect to the action time.
Regarding the antimicrobial action of gentamicin, of the 335 bacterial samples isolated, antibiotic test against gentamicin was performed in 305 samples and 88% (268/305) were sensitive to the antibiotic and 12% (37/305) resistant. Among the resistances, the Gram-positive bacteria are highlighted, as shown in Figure 4.
Discussion
Of the 30 corneal donors in this study, 63.3% were males, a percentage that is similar to other studies, which reported a rate of 62.2% and 61%, respectively [12,13], of corneal donor men and the age of the donors had a median of 54 years, data very similar to those described em others articles [14,15], and found 55 and 55.57 years of age, respectively.
The variables sex and age in this study did not interfere in the results found in relation to the times of 5, 10 and 15 minutes of action of the antiseptics for the reduction of the ocular surface microbiota, with p values found, p<0.999 for sex and the p<3.41 for age (Table 1), are not statistically significant.
Although the best result was detected in the 15 minutes group, the overall mean time between death and withdrawal from the eye was 5:15 minutes, which is in line with the EBAA recommendation, which is up to six to minimize metabolic changes, which can alter endothelial cells and microbiological contamination [16].
The cause of death was another variable of the present study and had the highest prevalence death due to acute myocardial infarction (data not shown). In the study by Araújo and Scarpi [17], the highest index (26%) of corneal donor deaths was also due to cardiovascular diseases. This high prevalence of causa mortis can be explained by the fact that cardiovascular diseases are the main cause of death in the world [18].
Staphylococcus spp. were the most prevalent (81.8%) and authors [19] have demonstrated that 63.8% of the strains found were Staphylococcus spp. and were the most isolated micro-organisms of the conjunctiva, eyelids and tears and are part of the microbiota of the eyes of living people [20]. These micro-organims, although considered to be of low virulence may be carried into the cornea preservation medium and subsequently transferred to the recipient, which may result in corneal transplant endophthalmitis.
Despite the low incidence, Gram-negative bacteria and fungi were also isolated. Studyes [6,17], in their studies found a rate of 41%/45.14%, respectively, of Gram negative bacteria, given slightly above this study; the high incidence of Gram-negative bacteria in cadaver eyes is due to the fact that donor eyes to be enucleated, often after the autopsy. This fact also occurred in the present study, with a longer period between death and enucleation in these donors [6].
The fungi were isolated from three donors who were hospitalized and one from the MDIS. In all, the incidence was 1.7% (4/353), being two Candida albicans. Although fungi are not considered to belong to the microflora of the ocular surface, they have been isolated at a rate of 28% in the eyes of healthy people [20]. In a previous study [21], authors described a rate of 0.5% of C. albicans, similar to the percentage found in this study, which was 1.3% of the total.
Study [22] reported that it is important to observe the risk factors that lead to the contamination of corneas donated with fungi. This contamination may occur due to diseases of the ocular surface or to the permanence in environments conducive to their growth [4]. For example, patients who have been hospitalized for many days with respirators are more prone to contamination ocular by fungi [23]. Another factor that can contribute is the type of environment, which can be hot and humid [7]. This explains the observed in this research, where two of the three donors who presented fungi were hospitalized for a long period and with respirator use.
In the present study, the significant reduction of the total microbiota of the ocular surface of donors of corneas observed for both povidone-iodine and gluconate of chlorhexidine occurred due to the broad spectrum of action that these antiseptics presented 53.6% decontamination (Table 4). Some works [24], in their study found a 36% reduction rate in the ocular surface microbiota, when performing antisepsis with 5% povidone-iodine solution for 2 minutes. With this procedure, the authors emphasized that the reduction of the number of microbial contaminants on the surface is significant, but does not totally eliminate the risk of contamination.
When evaluating the results obtained for Staphylococcus spp. and enterobacteria, with respect to antiseptic residence times and their effect on donor eye decontamination, although not statistically significant, both povidone-iodine and gluconate of chlorhexidine were more effective in 15 minutes time for Staphylococcus spp. (Figure 2), the effect of gluconate of chlorhexidine being higher than that of povidone-iodine. For the enterobacteria both were effective from 5 minutes (Figure 3). However, when the third collection was performed, on average 2:11 minutes after enucleation the rate of reduction of the microbiota was above 99.1% for the two antiseptics.
Some authors [25] used 5% povidone-iodine solution for 5 minutes in scarified corneas and found a significant reduction rate of 24.7% to 4.3%, but concluded that this concentration and this time of action of povidone-iodine significantly decreases the contamination of the corneal epithelium but does not completely sterilize the cornea and occasionally leaves a sufficient number of bacteria on the ocular surface to contaminate the preservation medium.
A work [6], in their antisepsis protocol, tested three different protocols, the first being gentamicin at 0.4%, the second gentamicin at 0.4% with povidone-iodine at 1%, and the third amicacin at 4 % with 5% povidone-iodine. In all treatments, the ocular globes were immersed for 3 minutes and the reduction of the microbiota was Gram-positive of 38.6%, 27.6% and 10.8% respectively, and for Gram-negative, 10.2%, 18.8% and 19.8%, respectively.
When comparing the results presented in the literature with those obtained in the present study, it is noted that the residual time of povidone-iodine and gluconate of chlorhexidine was the fundamental factor for the high rate of decontamination found, close to 100%. The next result of this study was that, when the authors [26] combined gluconate of chlorhexidine with povidone-iodine, obtaining reduction rate of 98.6%. The difference in methodologies was that, in addition to the combined use of povidone-iodine and gluconate of chlorhexidine, the preservation of corneal tissue occurred minutes after immersion in antiseptics. Already in this research, the antiseptics were used separately, irrigated before enucleation, within the established action time of each step. Next, the eyeballs were placed in a humid chamber and sent to eye bank for evaluation, processing and preservation, with an average duration of 2:11 minutes.
The observed growth of Staphylococcus spp. in the third collection from a single donor can be attributed to an extensive epithelial defect in both eyes, classified from (2++) to (3+++). Authors [27] aim that the damaged corneal epithelial tissue can retain micro-organisms in crypts and thereby protect them from the action of antiseptics during irrigation.
Regarding the antibiogram test, 88% were sensitive to gentamicin (Figure 4), with the highest resistance rate found for the group of Gram positive bacteria (7%). Others research [7,28], found an 82% sensitivity to the same antibiotic and 86.4%, respectively, which was similar to that detected in this study. This sensitivity rate is still of concern, because if there is adequate antisepsis of the ocular tissues prior to preservation, micro-organisms resistant to the antibiotic contained in the preservation medium may remain in the corneal tissue at the time of transplantation, resulting in an endophthalmitis in the recipient of the cornea.
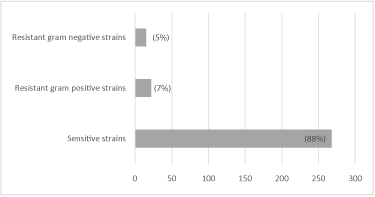
Figure 4: Percentage of gram-positive and gram negative bacterial strains
sensitive and resistant to gentamicin, isolated from the ocular surface of
donors of cornea.
This fact was observed in a study [29] a rate of 56.8% of cases of endophthalmitis after corneal transplantation, where the isolated micro-organisms were both in the corneal-scleral flap donor tissue, and in the eye of the recipient. In 2004, studies [30] described a much lower rate, 16% of contaminated corneal buds, of which only 1.5% caused infection in the recipient, which resulted in 1.27% of ulcer and 0.22% endophthalmitis.
Two groups of researchers [7] found that the gentamicin is the most effective antibiotic for the decontamination of donor eyes before enucleation and corneas preserved for transplantation, being the most used in the composition of commercial preservation media. In the present study, after the quarantine period and new reassessment of the preserved corneas, no vial presented turbidity and alteration in the color of the preservation medium, as indicative of pH change and possible contamination.
Thus, after evaluating the results obtained, it is believed that the use of strict antiseptic procedures, from removal of the eyeball to preservation, guarantees the safety of a corneal tissue to be used in transplants, which will reduce the risks of disorders after surgery.
There was no statistically significant difference between the action of povidone-iodine and gluconate of chlorhexidine in the reduction of the micro-organisms of the ocular surface of the cornea donors, both of which were effective. The time between removal of the eyeball and the preservation of the cornea allows the residual action of the antiseptics, which increases the decontamination power. Although some strains resistant to gentamicin have been found, the antibioticcontaining cornea preservation medium complements tissue decontamination procedures and provides greater storage safety.
Acknowledgement
The authors tank you the eyes bank by permit to study contamination using the donor corneas from eyes bank.
The author’s aims have no conflict of interest.
References
- Panda A, Angra SK, Kumar A. Microbial contamination of donor eyes. In: Adv in Corneal Res Springer US. 1997; 549-555.
- Hassan SS, Wilhelmus KR, Dahl P, Davis GC, Roberts RT, Ross KW, et al. Medical Review Subcommittee of the Eye Bank Association of America. Infectious disease risk factors of corneal graft donors. Arch of ophthalmol. 2008; 126: 235-239.
- Nogueira DC, Ueda SMY, Murça MAP, Hida WT, Felberg S, Hida RY et al. Comparação entre dois meios de coleta e transporte parba estudo da microbiota conjuntival de indivíduos normais. Arq Bras de Oftalmol. 2007; 70: 929-934.
- Merchant A, Zacks CM, Wilhemlmus K, Durand M, Dohlman CH. Candidal endophthalmitis after keratoplasty. Cornea. 2001; 20: 226-229.
- Barkana Y, Almer Z, Segal O, Lazarovitch Z, Ani I, Zadok D, et al. Reduction of conjunctival bacterial flora by povidone-iodine, ofloxacin and gluconate of chlorhexidine in an outpatient setting. Acta OphthalmolScand. 2005; 83: 360-363.
- Tandon R, Mehta M, Satpathy G, Titial JS, Sharma N, Vajpaee RB, et al. Microbiological profile of donor corneas: a retrospective study from an eye bank in north India. Cornea. 2008; 27: 80-87.
- Reddy SC, Paul G. Bacterial flora of conjunctiva after death. Intern J of ophthalmol. 2013; 6: 632.
- Basrani B. Gluconate of chlorhexidine gluconate. AustEndodc J. 2005; 31: 48-52.
- Jeng BH. Preserving the cornea: corneal storage media. Current opinion in Ophthalmol. 2006; 17: 332-337.
- Broniek G, Langwinska-Wosko E, Sybilska M, Szaflik JP, Wróblewska M. Prevalence of bacteria and fungi in samples of cornea preservation fluid. Arch of Medl Sci. 2016; 12.
- Kim KS, Edlhauser HF, Holley GP, Geroski DH, Lynn M, Walsh GE, et al. Corneal endothelial permeability of human tissue after storage in Optisol. Am J of Ophthalmol. 1994; 117: 385-393.
- Rootman DB, Wankiewicz E, Badmin LS, Baxter AS. In Situ Versus Whole- Globe Harvesting of Corneal Tissue from Remote Donor Sites-Effects on Initial Tissue Quality. Cornea. 2007; 26: 270-273.
- Linke SJ, Fricke OH, Edd MT, Bednarz J, Druchkiv V, Kaulfers PM, et al. Risk Factors for donor cornea contamination: retrospective analysis of 4546 procured córneas in a single eye bank. Cornea. 2013; 32: 141-148.
- Farias RJM, Kubokawa KM, Schirmer M, Sousa LBD. Avaliação de córneas doadoras em lâmpada de fenda e microscopia especular durante o período de armazenamento. ArqBras de Oftalmol. 2007; 70: 79-83.
- Sano RY, Sano FT, Dantas MCN, Lui ACF, Sano ME, Lui Neto A, et al. Análise das córneas do Banco de Olhos da Santa Casa de São Paulo utilizadas em transplantes. Arq Bras de of talmol. 2010; 73: 254-258.
- Wilson SE, Bourne WM. Corneal preservation. Surv of Ophthalmol. 1989; 33: 237-259.
- Araújo MEXS A, Scarpi MJ. Microbiota bacteriana da conjuntiva de doadores de córnea. ArqBras de Oftalmol. 2004; 67: 927-33.
- PAHO - Determinantes Sociais e Riscos para a Saúde, Doenças Crônicas não transmissíveis e Saúde Mental Doenças cardiovasculares – review in 2016 September setembro de 2016. Avaliable at http:/ /www.paho.org. Access in em16/08/2017.
- Keyhani K, Seedor JA, Shah MK, Terraciano AJ, Ritterband DC. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea. 2005; 24: 288-291.
- Badenoch PR, Alfrich SJ, Wedding TR, Cster DJ. Effectiveness of a decontamination method for donor corneas. British J of Ophthalmol. 1988; 72: 225-227.
- Rehany U, Balut G, lefler E, Rumelt S. The prevalence and risk factors for donor corneal Button contamination and its association with ocular infection after transplantation. Cornea. 2004; 23: 649-654.
- Willcox MDP. Characterization of the normal microbiota of the ocular surface. Exp eye res. 2013; 117: 99-105.
- Mindrup EA, Dubbel PA, Doughman DJ. Betadine decontamination of donor globes. Cornea. 1993; 12: 324-329.
- Pels E, Vrensen GFJM. Microbial decontamination of human donor eyes with povidone-iodine: penetration, toxicity, and effectiveness. Brit J of ophthalmol. 1999; 83: 1019-1026.
- Robert PY, Robert PY, Camezind P, Drouet M, Ploy MC, Adenis JP, et al. Internal and external contamination of donor corneas before in situ excision: bacterial risk factors in 93 donors. Graefe’s. Arch for clin and expophthalmol. 2002; 240: 265-270.
- Van Luijk CM, Bruinsma M, Van dWJ, Lie JT, Ham L, Melles GR, et al. Combined gluconate of chlorhexidine and PVP-I decontamination of human donor eyes prior to corneal preservation. Cell and tissue bank. 2012; 13: 333- 339.
- Isenberg SJ, Apt L, Yoshimori R, Khwarg S. Chemical preparation of the eye in ophthalmic surgery: IV. Comparison of povidone-iodine on the conjunctiva with a prophylactic antibiotic. Arch of ophthalmol. 1985; 103: 1340-1342.
- Pardos GJ, Gallagher MA. Microbial contamination of donor eyes: a retrospective study. Arch of Ophthalmol. 1982; 100: 1611-1613.
- Kloess PM, Stulting RD, Waring GO, Wilson LA. Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J of ophthalmol. 1993; 115: 309-316.
- Gopinathan U, Agrawal V, Sharma S, Rao GN. Donor corneoscleral rim contamination by gentamicin-resistant organisms. Indian J of ophthalmol. 1994; 42: 71.