
Special Article - Escherichia Coli
J Bacteriol Mycol. 2018; 5(1): 1059.
E. coli Coliform Mastitis in Doe and Its Antibiogram
Rudra PG1 and Dutta A2*
¹Department of Microbiology and Veterinary Public Health, Bangladesh
²Department of Veterinary Microbiology and Public Health, Chittagong Veterinary and Animal Sciences University, Bangladesh
*Corresponding author: Dutta A, Department of Veterinary Microbiology and Public Health, Chittagong Veterinary and Animal Sciences University, Bangladesh
Received: January 18, 2018; Accepted: February 13, 2018; Published: February 20, 2018
Abstract
Rearing of goat has an important impact in improving socio-economic status of rural people in Bangladesh. Coliform mastitis caused by Escherichia coli is one the predominant constraints of this livestock production in this country. This study was designed to investigate the situation of coliform mastitis by E. coli in clinically affected doe in Chittagong. A total of 29 milk samples of mastitic doe were collected. Confirmation of coliform mastitis by E. coli was done in MacConkey, EMB agars, biochemical and staining techniques. Later, isolates were tested against 7 antimicrobials of 5 different groups using disc-diffusion technique. Epidemiological data were analyzed using STATA software to reveal their association with occurrence of E. coli coliform mastitis. Out of 29 samples 8 were confirmed as E. coli. In relation to different host factors such as breed, age, body weight and parity the highest occurrence was observed in cross breed, doe of ≤ 2years, doe having body weight of >30kg, and in doe of 1st parity which was 50%, (95% CI 2.5%-78.4%), 28.6% (95% CI 7.6-64.8), 36.4% (95% CI 15%-64.8%), and 50% (95% CI 21.5%-78.45) respectively. E. coli isolates were highly sensitive to Gentamycin (75%) and Colisitn (75%). Highest resistance was recorded against Amoxicillin (100%) and Ciprofloxacin (100%). Statistical analysis using chi2 test showed that there is no significant relationship between host factors with the occurrence of E. coli coliform mastitis. Study suggests use of Gentamycin and Colistin for the treatment of coliform mastitis in doe caused by E. coli.
Keywords: Coliform Mastitis; E. coli; Parity; Doe
Introduction
Bangladesh is an agricultural country containing large number of domestic animals. Goat is one of them. Currently estimated goat population in Bangladesh is about 14.8 million (Banglapedia). This density has been increasing every year in the country. In agriculturebased country like Bangladesh goat is more familiar as poor man’s cow. Initial investment for starting goat farming is lesser than dairy, piggery, poultry and consumes less feed which is about one fifth of the consumption in cattle and buffalo [1]. In Bangladesh, goats are reared in only backyard farming system. Low income people are like to rear goat in their household for extra income. They prefer goat rearing than cattle because, it needs small scale space, low feeding cost, high litter size, low manpower for maintenance and meat has good market demand.
Goat farming in Bangladesh is very challenging due to many problems. Lack of financial and technical support, inadequate veterinary services are most crucial. Infectious diseases make the condition worse for goat rearing mainly viral diseases like PPR; Peste des Petits Ruminants, goat pox, contagious ecthyma and viral pneumonia, and bacterial diseases include enterotoxaemia, tetanus, brucellosis, mastitis and metritis whereas main fungal diseases are ring worm infection, and rickettial infections like conjunctivitis are common causes for goat mortality in rural areas. Gastro-intestinal nematodiasis, fascioliasis and tape worm infestations cause less mortality but cause severe depression in the growth and reproductive rate of the goats. Production disease such as mastitis, pregnancy toxemia, mineral deficiency also decreases production [2]. Common goat breeds reared in Bangladesh are: Black Bengal, JamunaPari, and Crossbreed- Black Bengal JamunaPari. More than 90% of the goats of the country are Black Bengal breed.
The advancement of goat farming in Bangladesh is interrupted by a number of constraints of which major one is mastitis. Mastitis is an inflammation of the parenchyma of mammary gland (udder). It is characterized by physical, chemical and usually, bacteriological changes in milk and pathological changes in glandular tissues [3].
The bedding used to house in animal is the primary source of environmental pathogens, but contaminated teat dips, intramammary infusions, water used for udder preparation before milking, water ponds or mud holes, skin lesions, teat trauma, and flies have all been incriminated as sources of infection [4,5].
Majority of coliform isolates from raw milk there were E. coli 32%, Enterobacter spp. 29.2%, Klebsiella spp. 19.4%, Serratia spp. 11.1% and Citrobacter 1.0% [6]. Coliform bacteria causes as many as 30-40% of the clinical mastitis in farm. Escherichia coli, Enterobacter aerogenes, Klebsiella pneumonia and Serratia marcesans are four common coliform bacteria that cause mastitis. Among the coliform mastitis only E. coli cause 5.719% mastitis in goat.
The most common bacteria that cause mastitis in goats are: Staphylococcus aureus, coagulase- negative Staphylococci spp., Streptococcus agalactiae, Streptococcus uberi, Streptococcus dysgalactia, Streptococcus caprae, Mycoplasma capricolum, Enterobacteria spp, Escherichia coli, Pseudomonas aeruginosa, Clostridium spp etc. The Caprine Arthritis-Encephalitis Virus (CAEV) causes mastitis in goats. In addition, mastitis can result from yeast infection, and it appears to be associated with the frequent use of penicillin, along with the prolonged and repetitive use of systemic and intra-mammary infusions.
Escherichia coli are a gram negative, non-spore forming rod. It may or may not be mobile. (Some rods are flagellated and some are not.) The organism is a facultative anaerobe and ferments simple sugars such as glucose to form lactic, acetic, and formic acids. The optimal conditions for growth are a temperature of 98.6°F, with a range of 45° to 114°F. The optimum pH for growth is 6.0 to 8.0. However, growth can occur as low as pH 4.3 and as high as 9 to 10 pH.
Bacteriological culturing of the milk can be used to determine if mastitis is caused by E. coli coliform bacteria. Amoxicillim, Ceftiofur, Penicilln, Cephapirim, Ciprofloxacin are those types of systemic antibiotic which can be used in coliform mastitis. In Bangladesh numerous antibiotics are used in field condition for treating the mastitis in goat.According to commonly used antimicrobilals are Gentamycin, Gentamycin+Amoxicillin, Amoxicillin, Benzyl- Penicillin, Streptomycin+ Procaine Penicillin, Ceftriaxone, Sulphadimidine, Gentamycin+ Sulphadimidine+ Trimethoprim, Gentamycin+ Amoxicillin+ Sulphadimidine, Tetracycline.
There is very little or no published information regarding the occurrence of clinical mastitis in goat caused by E. coli in Chittagong, Bangladesh. Considering the social and economic importance of goat rearing in Bangladesh present study was undertaken to reveal the present scenario in Chittagong.
The objectives of the present study are
1. To know the prevalence of coliform mastitis caused by E. coli in clinically affected goat.
2. To investigate the antimicrobials sensitivity patterns of E. coli that causing mastitis in goat.
3. To reveal the association of risk factors with the occurrence of coliform mastitis.
Materials and Methods
Study area and duration
The study was conducted during the period of 22nd January to 6th April; 2017. About 29 milk samples were collected from the doe which were suffering from clinical mastitis. Most of the samples were collected during UVH; Upazilla Veterinary Hospital placement of internship. Eighten samples from UVH; Upazilla Veterinary Hospital, Hathazari, seven samples from SAQTVH; S.A. Quaderi Teaching Veterinary Hospital, CVASU; Chittagong Veterinary and Animal Sciences University during the period of lab rotation and another four samples from Rangunia Veterinary Hospital, Rangunia. Geographical location is mentioning in the Figure 1.
Figure 1: Geographical location of study area.
Sample collection
The samples were collected from mastitis infected quarter(s) of the mammary gland through hand milking following aseptic procedure and immediately transferred into eppendorf tube. Samples were transported from the collection site to Microbiology Laboratory, CVASU maintaining cool chain for detailed analysis. Collected samples were preserved in a refrigerator at 4°C until screening out the bacteria.
Data collection
Data were collected during sample collection using a pre-designed questionnaire. Collected data include basic information regarding the animals (breed, age, parity, lactation period, body weight, litter size, previous mastitis history etc) and records on treatment of mastitis. Owner’s contact details were collected to follow up the cases.
Isolation and identification of E. coli
For the isolation of E. coli from each collected milk samples at first 1ml milk sample was inoculated into the test tube containing buffer peptone water (BPW) (Oxoid Ltd, PH: 6.2± 0.0, Basingstoke, Hampshire, UK) and incubated at 37°C overnight for primary enrichment. After primary enrichment the culture was streaked on MacConkey agar medium (Oxoid Ltd, PH: 7.4± 0.2, Basingstoke, Hampsshire, UK) and incubated at 37°C for 24 hours. Culture of the sample produced bright pink colored, large and non-mucoid colonies on MacConkey agar. The organism was suspected as E. coli based on colony morphology. Later individual colony from MacConkey agar was streaked on EMB; Eosin Methylene Blue agar plate (Merck, PH: 7.1± 0.2) and incubated at 37°C for 24 hours. Based on “green metallic sheen” colony morphology the organism was confirmed as E. coli along with following biochemical and Gram’s staining properties. Isolated E. coli on EMB is shown in the Figure 2.
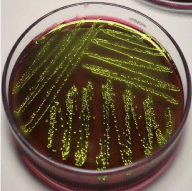
Figure 2: E. coli on EMB agar.
Preservation of isolates
All E. coli isolates were cultured in BHI; Brain Heart Infusion broth, incubated overnight at 37°C. For each isolate 700μl BHI broth culture was add to 300μl 15% glycerol in an eppendorf tube. Tubes were properly leveled and stored at -80°C for further investigation.
Screening of the antimicrobial sensitivity pattern of the isolates using a panel of antimicrobials
The antimicrobials commonly used for treatment of clinical cases of goat in field condition especially for mastitis was included in the CS; cultural sensitivity test. Details about the antimicrobials along with their interpretation are summarized in Table 1.
Microbial group
Anti-microbial agent
Disc potency
Zone Diameter (mm)
Sensitive
Intermediate
Resistant
Β-lactams antibiotics
Amoxicillin (AML)
10μg
≥17
14-16
≤13
Ceftriaxone (3rd Generation cephalosporin) (CRO)
30μg
≥18
15- 18
≤14
Cefotaxime (3rd Generation cephalosporin) (CTX)
30μg
≥ 18
15-18
≤14
Macrolides
Gentamycin (CN)
10μg
≥15
13-14
≤12
Polymixin
Colistin Sulphate (CT)
8μg
≥11
≤10
Aminoglycosides
Streptomycin(S)
10μg
≥19
15-18
≤14
Quinolones
Ciprofloxacin (CIP)
5μg
≥21
17-20
≤16
Table 1: Concentrations and diffusion zone breakpoints for resistance against anti-microbial’ standard for E. coli isolates (CLSI. 2011).
Procedure of cultural sensitivity (CS) test
At first sub-culturing of the preserved organism was done on blood agar and incubated at 37° for 24 hours to obtain a pure growth. Using sterile inoculating loop 3 or 4 individual colonies from the blood agar were transferred into a tube containing 3ml of sterile phosphate buffer saline solution (0.85% w/v NaCl solution). Emulsification of the inoculums was done to avoid clumping of the cells inside test tube using vortex machine. Then the bacterial suspension was adjusted to the turbidity of 0.5 McFarland standards (equivalent to growth of 1-2×108 CFU/ml). Within 15 minutes of preparing the inoculums, a pre-sterile cotton swab was dipped into the Inoculums and rotated against the side of the tube with firm pressure to remove excess fluid. Then the swab was streaked over the entire dry surface of Mueller Hinton agar for three times rotating the plate approximately at 60 degrees. After 15 minutes of inoculation the discs were placed on the agar surface using a sterile forceps. After dispensing all the discs the agar plates were incubated at 37°C for 18 hours. After incubation the size of zone of inhibition (in mm) around a disc including the diameter of the disc was measured using a ruler and the result was interpreted according to CLSI; Clinical and Lab Standard Institute, 2011. Zone of inhibition shown in the below mention Figure 3.
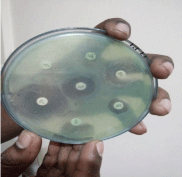
Figure 3: Zone of inhibition.
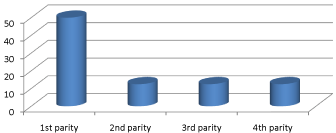
Figure 4: Occurrence of coliform mastitis in doe relation to parity.
Data analysis
The antimicrobial susceptibility data are expressed as percentages or frequency of the E. coli isolated from mastitis effected goat milk. During conducting the study epidemiological data were collected to explore their association with occurrence of coliform mastitis in goat. The epidemiological data included breed, age, parity, lactation period, body weight, litter size, previous mastitis history. All data were inputted into a spreadsheet (Microsoft Office Excel 2010) and transferred to STATA-11 for statistical analysis. The tests were conducted at 95% level of confidence and 5% level of significance. The p value less than 0.05 were considered statistically significant.
Results and Discussion
Prevalence of E. coli coliform mastitis
Out of 29 samples collected from mastitis affected goat, 8 samples were found positive for E. coli. Table 2 shows the overall prevalence of E. coli in mastitis affected doe. Prevalence of E. coli mastitis in doe is 27.6% (95% CI; Confidence Interval, 14.5% - 45.9%)
Total samples no
E. coli positive samples no
Prevalence (%)
29
8
27.6 (95% CI 14.5% - 45.9%)
CI: Confidence Interval
Table 2: Prevalence of coliform mastitis caused by E. coli.
Prevalence of E. coli coliform mastitis relation to the breed of doe
The results of occurrence of E. coli causing coliform mastitis in relation to breed of the animals are shown in Table 3. Here, it’s seen that highest (50%; 95% CI 21.5%-78.4%) occurrence was recorded in cross breed goats.
Name of the breed
No of mastitis effected doe
Percentage (%)
Cross breed
4
50% (95% CI 21.5%-78.4%)
Jamunapari
1
12.5% (95% CI 1.1%-49.2%)
Black Bengal
3
37.5% (95% CI 13.4%-69.6%)
Total
8
100
Table 3: Occurrence of E. coli coliform mastitis in doe relation to the breed of animal.
Occurrence of E. coli coliform mastitis in relation to age
The results of occurrence of E. coli causing coliform mastitis in relation to age of the animals are shown in Table 4. Here it is seen that, highest (28.6 %, CI 7.6% - 64.8%) occurrence was recorded in age of two years or less than two years.
Categories
No of samples (positive sample no)
Prevalence (%) (95% CI)
= 2 years
7 (2)
28.6 (7.6 - 64.8)
> 2 years
22 (6)
27.8 (10.7 - 50.2)
Table 4: Occurrence of E. coli coliform mastitis in relation to age.
Occurrence of E. coli coliform mastitis in relation to body weight
The results of occurrence of E. coli causing coliform mastitis in relation to weight of the animals are shown in (Table 5). Here it is seen that, highest (36.4%, CI 15% - 64.8%) occurrence was recorded in the doe which weight over 30kg.
Categories
No of samples (positive sample no)
Prevalence (%) (95% CI)
= 30 kg
18 (4)
22.2 (8.5 - 45.8)
> 30 kg
11 (4)
36.4 (15 - 64.8)
Table 5: Occurrence of E. coli coliform mastitis in relation to body weight.
Occurrence of coliform mastitis according to the parity of doe
The results of occurrence of E. coli causing coliform mastitis in relation to parity of the animals are shown in Table 6. Here, it’s seen that highest (50%; 95% CI 21.5%-78.4%) occurrence was recorded in 1st parity goats.
Antimicrobials
Antimicrobial resistance pattern(95% Confidence Interval, CI)
Sensitive (%)
Intermediate (%)
Resistant (%)
AML
0%
0%
100% (62.8%-100%)
CN
75% (40%-93.7%)
12.5% (0.1%-49.2%)
12.5% (0.1%-49.2%)
CT
75 % (40%-93.7%)
0%
25% (6.3%-59.9%)
S
37.5 % (30.5%-69.6%)
12.5 % (0.1%-49.2%)
50% (21.5%-78.5%)
CIP
0%
0%
100% (62.8%-100%)
CRO
37.5 % (13.5%-69.6%)
12.5 % (0.1%-49.2%)
50% (21.5%-78.5%)
CTX
37.5 % (13.5%-69.6%)
0%
62.5% (3.4%-86.5%)
Table 6: Antimicrobial resistance profiles of the E. coli isolates.
Antimicrobial sensitivity profiles of bacterial isolates
The results of antimicrobial sensitivity pattern of the E. coli isolates against seven antimicrobials tested are shown in Table 7.
Variables
No of samples
No of positive sample for coliform mastitis
Chi square value
P value
Breed
Cross breed
12
4
1.13
0.567
Jamunapari
2
1
Black bengal
15
3
Age group
= 2 years
7
2
0.005
0.947
> 2 years
22
6
Body weight
= 30 kg
18
4
0.684
0.408
> 30 kg
11
4
Parity
1st
10
5
4.118
0.249
2nd
17
3
3rd
1
0
4th
1
0
Treatment
No antibiotics
2
0
4.738
0.094
One antibiotic
16
7
Combined antibiotics
11
1
Recovery status
Yes
9
3
0.514
0.773
No
6
2
Unknown
14
3
Table 7: Statistical analysis of the host factors with the occurrence of coliform mastitis of doe by E. coli.
Association of host factors with the occurrence of E. coli coliform mastitis in doe
Statistical analysis of the epidemiological data of host factors and the treatment strategies followed to treat the clinical cases showed no statistical significant association with the E. coli coliform mastitis. Results of the statistical analysis are summarized in Table 7.
The aim of this study was to focus on isolation and identification of the E. coli from coliform bacteria in clinically effected mastitic doe and want to know their antimicrobial pattern, which one more successful against E. coli. Eight (27.6%) milk samples were positive for E. coli in this study (Table 3) and are in close agreement with that of [7] in eastern [8] in Kano State, Nigeria. However, higher prevalence of coliform mastitis were reported by [9] in India, [10] in Southwestern Ethiopia and in Jordan by [11]. The isolated genus of coliform bacteria in this study only was E. coli (Table 3). This is in close agreement with [4,7,8,12-14] Who all found Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter, Serratia and Proteus as major mastitogens. Cross breed goats are produce more milk than Black Bengal goat which is agreed with [3] stated that high yielding animals are more susceptible to mastitis than low-yielding ones.
Antimicrobial susceptibility testing of E. coli isolates from coliform organisms showed that, all E. coli isolates were sensitive to Gentamycin and Colistin Sulphate followed by decreasing susceptibilities to streptomycin, Ceftriaxone, Cefotaxime, Ciprofloxacin and Amoxicillin Table 3. The pattern of susceptibility and resistance exhibited in the present study may be due to prolonged and indiscriminate usage and prescription of particular antibiotics which often leads to possible resistance development in animals [15- 17].
Conclusion
Mastitis is one of the most important constrains in goat farming in Bangladesh. E. coli is found to be the main organism that causes the coliform mastitis in clinically affected mastitic doe. Gentamycin and Colistin Sulphate are the two most effective antimicrobials for clinical management of the clinical mastitis in goat.
References
- Das SK. Prospect and potentiality for goat farming in north eastern region of India-A review. Agriculture reviews-Agricultural Research Comminucations Center India. 2001; 22: 228-233.
- Kashem MA, Hossain MA, Ahmed SU, Halim MA. Prevalence of diseases, morbidity and mortality of Black Bengal Goats under different management systems in Bangladesh. University Journal of Zoology, Rajshahi University. 2012; 30: 1-4.
- Radostitis OM, Blood DC, Gay CC. In: Veterinary Medicine, A text book of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. ELBS. 2000: 241-248.
- Matofari JW, Mario Y, Mwatha EW, Okemo PO. Microorganisms associated with sub-clinical Mastitis in the Kenyan Camel (Camelusdromedarius). Journal of Tropical Microbiology and Biotechnology. 2003; 2: 11-16.
- Kivaria FM, Noordhuizen JP, Msami HM. Risk factors associated with the incidence rate of clinical mastitis in smallholder dairy cows in the Dares Salaam region of Tanzania. The Veterinary Journal. 2007; 173: 623-629.
- Salman AM, Hamad IM. Enumeration and identification of coliform bacteria from raw milk in Khartoum State, Sudan. Journal of Cell and Animal Biology. 2011; 5: 121-128.
- Abdurahman OA. Udder health and milk quality among camels in the Errer valley of eastern Ethiopia. Livestock Research for Rural Development. 2006; 18: 3-11.
- Kalla DJU, Butswat ISR, Mbap ST, Abdussamad AM, Ahmed MS, Okonkwo I, et al. Microbiological Examination of camel (Camelusdromedarius) milk and sensitivity of milk microflora to commonly available antibiotics in Kano. Nigeria Sav. J. Agric. 2008; 3: 1-8.
- Sena DS, Mal G, Kumar R, Sahani MS. A preliminary study of prevalence of mastitis in camel. Journal of Applied Animal Research. 2001; 20: 27-31.
- Woubit S, Bayleyegn M, Bonnet P, Jean-Baptiste S. Camel (Camelusdromedarius) mastitis in Borena, lowland pastoral area, southwestern Ethiopia= Mamites du dromadaire (Camelusdromedarius) dans la régionpastoralebasse du Borana au sud-ouest de l’Ethiopie= Mastitis en camellas (Camelusdromedarius) en la zonapastorilbaja de Borena, sudoeste de Etiopia. Revue d’élevageet de médecinevétérinaire des pays tropicaux. 2001; 54.
- Hawari AD, Hassawi DS. Mastitis in one humped she-camels (Camelusdromedarius) in Jordan. J Biological Sci. 2008; 8: 958-961.
- Giannino ML, Marzotto M, Dellaglio F, Feligini M. Study of microbial diversity in raw milk and fresh curd used for Fontina cheese production by cultureindependent methods. International journal of food microbiology. 2009; 130: 188-195.
- Abera M, Abdi O, Abunna F, Megersa B. Udder health problems and major bacterial causes of camel mastitis in Jijiga, Eastern Ethiopia: implication for impacting food security. Tropical animal health and production. 2010; 42: 341-347.
- Garedew L, Berhanu A, Mengesha D, Tsegay G. Identification of gramnegative bacteria from critical control points of raw and pasteurized cow milk consumed at Gondar town and its suburbs, Ethiopia. BMC Public Health. 2012; 12: 950.
- Sharma M, Dogra BB, Misra R, Gandham N, Sardar M, Jadhav S, et al. Multidrug resistant Pantoea agglomerans in a patient with septic arthritis-a rare report from India. International Journal of Microbiology Research. 2012; 4: 263-265.
- Koop G, Islam MN, Rahman MM, Khatun M, Ferdous J, Sayeed MA, et al. Risk factors and therapy for goat mastitis in a hospital-based case-control study in Bangladesh. Preventive veterinary medicine. 2016; 124: 52-57.
- Mbuk EU, Kwaga JKP, Bale JO, Boro LA, Umoh JU. Coliform organisms associated with milk of cows with mastitis and their sensitivity to commonly available antibiotics in Kaduna State, Nigeria. Journal of Veterinary Medicine and Animal Health. 2016; 8: 228-236.