
Research Article
J Bacteriol Mycol. 2018; 5(1): 1061.
Morpho-Physiological Diversity of Root Nodule Rhizobia from Mimosa (Mimosa pudica L) and Water Mimosa (Neptunia oleracea L)
Rahman MH1, Khatun S1, Ali SR1, Yasmin S1, Kamruzzaman M2 and Rashid MH3*
¹Department of Biotechnology, Bangladesh Agricultural University, Mymensingh, Bangladesh
²Plant Breeding Division, Bangladesh Institute of Nuclear Agriculture, Mymensingh, Bangladesh
³Department of Biology, Bangladesh Institute of Nuclear Agriculture, Mymensingh, Bangladesh
*Corresponding author: Rashid MH, Senior Scientific Officer, Biotechnology Division, Bangladesh Institute of Nuclear Agriculture, Mymensingh, Bangladesh
Received: January 22, 2018; Accepted: February 16, 2018; Published: February 23, 2018
Abstract
Legume genera Mimosa and its root nodulating rhizobia are studied recently by many researchers around the world due to its unique symbiotic relationship with nitrogen fixing bacteria which reduces the requirements of nitrogen during their growth. Considering the scarcity of information on rhizobia from mimosa from Bangladesh, we studied diversity of rhizobia associated with two mimosa species growing at dry land and wet land in Bangladesh. Twenty two (22) strains from root nodules of dry land mimosa and water mimosa from five different districts of Bangladesh and two strains from Malaysia were isolated and characterized on the basis of their growth, morphological and biochemical properties. A diverse characteristics were found in morphological (colony size, shape, color) and physiological (response to BTB reaction, salt, pH and temperature tolerance) characteristics. Their colony size was 1.9-3.5mm and color varied from transparent to milky white; produced green, light blue and blue color in response to BTB reaction; salinity (NaCl) tolerance up to 2.5% (MB-17, MB-22, MB-26, MB-32 and MB-33); growth at highly acidic (pH 4.0; MB-17, MB-32, MB-33, MB-43, MB-47, MB-48, MB-49) medium. The study showed that rhizobial strains from dry land mimosa grown well at highly acidic and saline condition while rhizobia from water mimosa grown well at more alkaline condition. Among 24 strains, MB-17, MB-32, MB-33 and MM- 54 showed resistance to high temperature (43oC). However, rhizobia from dry land mimosa were very specific for nodule formation only with their original host while rhizobia from water mimosa have broader host range to nodulate water mimosa and giant mimosa. Interestingly, two strains (MB-64 and MB-65) from water mimosa were availed to form nodules with three types of mimosa and might have diverse nodulation genes for nodulating a wide range of mimosa. Moreover, in pot experiment the strains MB-49 and MB-66 produced the highest nodules and dry matter weight in dry land and wet land mimosa over control treatment, respectively. This study helps us to know the diversity of mimosa nodulating rhizobia which will extend their application in agriculture practices, biotechnological application and alleviation of salt stress affected soils.
Keywords: Rhizobia; Mimosa; Stress Tolerance; Diversity
Introduction
Nitrogen (N2) fixation is an ancient prokaryotic trait that predates plant evolution [30]. Some bacteria contains nitrogenase enzyme to assimilate atmospheric nitrogen. The rhizobial enzyme system supplies a constant source of reduced N2 to the host plant and plant furnishes nutrients and energy for the activities of the bacterium. The malate (a breakdown product of sucrose) is the direct carbon source for the bacteroid. The legume plants are capable to produce root nodules which make them an ideal agricultural organism. Within legume nodules, atmospheric N2 is converted into ammonia, which is then assimilated into amino acids, nucleotides, and other cellular constituents. Mimosa poses a unique symbiotic relationship with nitrogen fixing bacteria which reduces the requirements of nitrogen during their growth [8].
Mimosa (Mimosa pudica L.) is a creeping annual or perennial herb, commonly found on cultivated lands or road side of Bangladesh.
It contains alkaloids, non-protein amino acid (mimosine), flavonoids, C-glycosides, sterols, terpenoids, tannins, fatty acids, a-spinasterol and phenyl ethylamine derivatives [17]. Mimosa exhibits various anti-bacterial, anti-depressant, anti-estrogenic, anti-implantation, vibriocidal, aphrodisiac, sinus, urolithiasis and hypolipidemic activity [11,20,31,32]. Mimosa root symbionts are also well-known metal-resistant bacteria [7,28]. Another leguminous plant, water mimosa (Neptunia oleracea L.) has been extensively used in phytoremediation to treat heavy metals (Cd, Cu, Zn, Pb, and Mn), and soluble solids [15,27,33]. Moreover, it efficiently reduces the biological and chemical oxygen demands [33]. Water mimosa is a good source of minerals and vitamins [34]. It exhibits antimicrobial and anticancer properties [4].
Specific strains of rhizobia are required to make functional nodules on the roots of legumes as they can promote growth of the host plants by increasing supply or availability of the primary nutrients, through the natural processes by fixing N2 or via solubilizing phosphorus [29]. Rhizobia are now important component of integrated nutrient management. They are relatively safer, environmentally friendly and cost-effective and an alternative to reduce chemical fertilizer. The rhizobial strains from mimosa have significant effects to increase ‘seedling height’ and ‘total dry weight’ at the later stage of rice [29]. But the selection of bacterial strains with multiple beneficial characteristics is important to maximize the effectiveness on the host plant [16]. It’s important to study the diversity of root nodulating rhizobia of mimosa and water mimosa from Bangladesh to get maximum benefit. Their isolation and characterization might provide a platform for their use in agricultural practice, crop improvement and future research. Therefore, present study was undertaken to assess the morpho-physiological diversity of mimosa and water mimosa root nodulating rhizobia and to identify potential strains for agricultural and industrial use.
Materials and Methods
Nodule collection
The dry land mimosa root nodules were collected from naturally grown mimosa plants from fallow-lands and road sides of four different districts of Bangladesh (Khulna, Magura, Mymensingh and Satkhira). Water mimosa root nodules were collected from naturally grown water mimosa on pond at ‘Sherpur’ districts of Bangladesh. Two strains were isolated from Bangi, Malaysia. Nodules from five plants from each site were collected and preserved in silica gel for further use. Well developed, uninjured, round shape and pink colored healthy nodules were chosen for bacterial isolation purposes.
Surface sterilization of nodules
Collected nodules from silica gel were soaked in sterile water for 5 hours at room temperature before processing for rhizobial isolation purpose. Then nodules were washed with 70% ethanol for 1 minute followed by 3min washing with 2% NaOCl. Then, nodules were washed 7 times with sterile distilled water (dH2O) to remove extra surface disinfectant.
Isolation of rhizobia from nodules
After surface sterilizing, nodules were crushed individually in eppendorf tubes containing 50µL sterile dH2O by sterile micro homogenizer. Subsequently, one full loop of suspension was picked from crashed nodule containing bacteria, and spread smoothly on Congo red yeast extract mannitol agar (CRYEMA) media in Petridish. Then inoculated plates were incubated at 280C for 72 hours. Cultures were purified by repeated streaking on CRYEMA plates to get pure single colony.
Preservation of isolated rhizobial strains
Single colony was preserved in agar slant at 40C for working sample. For long term preservation of the isolates, 5mL of fresh liquid culture of bacteria was taken into the equal volume of 50% glycerol and stored at -800C in 2mL eppendorf tube.
Colony size measurements of isolated strains
Colony size of the strains was measured using millimeter graph paper. A single loop of bacterial culture was streaked on a CRYEMA containing plate sequentially like cord of circle from one edge to another and incubated at 280C. After 3 days, using millimeter graph paper on plates keeping just opposite site of colonies, the diameters of them were measured. Mean value was taken from each strain of five colonies.
Nodulation and cross inoculation test
Mimosa, water mimosa and giant mimosa seeds were collected from field grown plant for nodulation and cross inoculation tests. Collected seeds were taken in 15mL Falcon tubes and washed with flooded sulfuric acids (H2SO4, 98%) for 3 minutes and then the seeds were washed with sterile water. Afterward, 2% NaOCl were added to the seeds and shaking for 10 minutes. Later, the seeds were washed for seven times with dH2O via vortex to remove excess surface disinfectant. Finally, these seeds were immersed overnight in sterilized dH2O in that tube. Overnight water-immersed sterilized seeds were transferred to water-Agar medium in a Petridis for germination in dark for three days at 370C. Germinated seedlings were then transferred to 150mL conical flasks/test tubes containing Fahraeus-nitrogen free medium. After two weeks of plant transfer at plant growth room, three types of mimosa (dry land mimosa, water mimosa and giant mimosa) plants were inoculated with 2mL of each overnight growth bacterial culture. To know the host range, each strain was used to inoculate three types of mimosa (dry land, water and giant). Three replications for each strain were maintained and every flask was labeled according to strain name and date of inoculation. Nodulations were observed after four to five weeks of inoculation. A 16/8 hrs light/dark photoperiods were maintained at growth chamber and plants were irrigated with 0.5X Fahraeus N-free nutrients medium at 7 days intervals for five weeks.
Morpho-physiological characterization of isolated rhizobial strains
In case of bromo-thymol blue (BTB) test, YEMA medium were prepared containing 10mL/L BTB solution in YEMA. For salt tolerance test, media were prepared with 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0% (w/v) NaCl containing YEMA, in conical flax. For pH tolerance test, four different levels of pH (pH4.0, pH5.0, pH9.0 and pH10.0) were made where the pH was controlled either by adding 1M acetic acid or 1M sodium hydroxide to make YEMA media acidic or basic. For temperature tolerance test, only YEMA media were used but inoculated plates were incubated at different temperature. One µL of each overnight grown rhizobial culture was dropped on labeled squares in petri-plates by micropipette. Three replications were maintained for accurate result. Until the drops were absorbed by the solid media, the petri-dishes wrapped with paraflim. Finally, plates were incubated at 280C for 3 days except culture plates for temperature tolerance test were incubated at 10C, 40C, 310C, 340C, 370C, 400C, 430C and 460C.
Collection and preservation of mimosa seeds for plant infection and symbiotic affectivity test
Mature, healthy and fresh seeds of mimosa and water mimosa were collected from naturally grown plants during October to December in 2015 to conduct plant infection and pot experiment. The collected seeds were dried under sun-light, and then stored in screw caped glass bottle in room temperature for further use.
Symbiotic effectiveness of isolated rhizobia on host plant growth
Each pot contained 2.00Kg soil, mixed with some chemical fertilizer, with TSP (12.38gm), MoP (9.52gm), ZYP (7.73gm), ZnSO4 (0.42gm), H3BO3 (0.45gm). Mimosa seeds were prepared by 10 minutes acidic treatment (98% H2SO4), then 7 times washing with water. Followed by overnight soaked in water; moisture containing viable seeds (4 seeds per pot) were sowed in pots and cultivated as usual. After 2 weeks of sowing, plants were inoculated with bacterial cultures (3mL per plant). Beside bacterial treatment a treatment of urea and a control were kept in the study. Each treatment implemented with 5 replications.
Plant harvesting, dry weight measurement and data analysis
After six weeks of inoculation, the plants were harvested, washed and nodules were separated from the roots. The nodules were separated from plants, counted and data were recorded. Then nodules were preserved in paper packets and plants were kept in brown paper packets and both plants and nodules were dried at 650C for 72 hours in hot air oven, slightly modified from Laguerre et al. [13]. After drying properly the weight of dry mass was measured by electric balance. The data were analyzed using MSTAT-Program and the means were compared using ANOVA at 1% level of statistical significance. The compared effectiveness of each treatment were evaluated through Duncan’s, Multiple Range Test (DMRT) by comparing the mean values of different characters [9] and the results are shown in (Table 5,6).
Results and Discussion
Nodulation and cross inoculation test
In vitro nodulation test was performed on respective hosts (dry land mimosa and water mimosa) to confirm the nodulation nature of our collected strains. We evaluated 24 strains of which 21 strains produced nodule in vitro in their respective hosts (Figure 1). All strains were availed to form nodules with their respective host at laboratory conditions except the strain MB-22 and MB-59 (Table 1). These are possible for opportunistic bacteria which enter insides the nodules with the help of other nodule forming bacteria at field conditions. Thus, the strains MB-22 and MB-59 might be opportunistic bacteria entered into the nodules of mimosa at field conditions with nodule forming rhizobia [24]. Thus, they were unable to form nodules at laboratory conditions. Interestingly, two strains MB-64 and MB-65 were availed to form nodules with dry land and giant mimosa in additions to their respective host suggesting that they had broad host range for nodulation and might have different nodulation genes than other strains from water mimosa.
Isolates/
strain name
Host plants
Dry land mimosa
Water mimosa
Giant mimosa
Nodulation capability
Numbers of nodules
Nodulation capability
Numbers of nodules
Nodulation capability
Numbers of nodules
MB-08
+
12
-
0
-
0
MB-17
+
13
-
0
-
0
MB-22
-
0
-
0
-
0
MB-26
+
8
-
0
-
0
MB-32
+
4
-
0
-
0
MB-33
+
3
-
0
-
0
MB-43
+
8
-
0
-
0
MM-46
+
4
-
0
-
0
MB-47
+
11
-
0
-
0
MB-48
+
10
-
0
-
0
MB-49
+
7
-
0
-
0
MM-54
+
5
-
0
-
0
MB-56
+
3
-
0
-
0
MB-57
-
0
+
7
+
6
MB-58
-
0
+
12
+
2
MB-59
-
0
-
0
-
0
MB-60
-
0
+
9
+
6
MB-61
-
0
+
3
+
2
MB-62
-
0
+
4
+
2
MB-63
-
0
+
6
+
2
MB-64
+
4
+
9
+
6
MB-65
+
3
+
8
+
4
MB-66
-
0
+
5
-
0
MB-67
-
0
+
8
+
2
Control
-
0
-
0
-
0
Table 1: In vitro nodulation test and host range recognition of collected isolates.

Figure 1: In vitro growing plants from sterilized seeds for nodulation test.
Abbreviations: A: Germination Efficiency Test of Collected Seed; B:
Sterilized Mimosa Seed Germinated after 3-4 Days Incubation at 370C; C:
Germinated Giant Mimosa Plant; D: Inoculated Mimosa Plant at Growth
Chamber; E: Inoculated Water Mimosa; F: Inoculated Giant Mimosa.
Colony morphology of the isolated strains
Most of the strains on the CRYEMA medium were milky white to creamy colored, where some of them were transparent. Most of the mimosa root symbionts appeared milky white (MB-08, MB-32, MB-33, MB-43, MB-47, MB-48, MB-49 and MB-56), but few of them produced transparent colonies (i.e. MB-17, MB-22, MB-26, MM-46 and MM-54). All water mimosa root symbionts (MB-57, MB-58, MB-59, MB-60, MB-61, MB-62, MB-63, MB-64, MB-65, MB-66 and MB-67) produced milky white colonies (Table 1) which are similar to other studies [3,23]. The study found colony size ranged from 2.2 to 3.5 mm in case of mimosa root symbionts; where the smallest was MB-56 and the largest was MM-54. The water mimosa nodulating rhizibial colonies were ranges from 1.8mm (MB-64) to 2.8mm (MB-58 and MB-60). We found bigger colony size from our strains (maximum 3.5mm) then the other studies who have found the maximum colony size of 2mm [5]. We found diversified colony characters; transparent to milky white in color, round shaped, convex and moderately mucoid with smooth edge. Similar to our study a diverse colony characters like circular, convex and domed shape with entire margins; and mucous producers were also found by others [5,6].
Characterization of isolated root nodulating strains based on acid-alkali production
In our present investigation, strains showed green to light blue colors (Figure 2), after 3 days of incubation at 280C on YEMA-BTB plates. We found that, 8 mimosa strains (MB-17, MB-32, MB-33, MB-43, MB-47, MB-48, MB-49 and MB-56) form green color; which represents acidic reaction. The remaining 4 strains viz. MB-08, MB- 22, MB-26 and MM-54 form light blue color, which indicates alkaline reaction producer. On the other hand, all tested water mimosa root symbionts (MB-57, MB-58, MB-59, MB-60, MB-61, MB-62, MB-63, MB-64, MB-65, MB-66 and MB-67) produce deep blue color (Figure 2) on YEMA-BTB plates; formation of deep blue indicates these strains exhibit alkaline reaction. Suggesting that rhizobial strains found from dry land mimosa were both first and slow grower while rhizobial strains from water mimosa were slow grower because, the colonies of fast growers produced acid reactions, whereas the colonies of the slow growers produced alkaline reactions in YEMA-BTB medium [23,24].
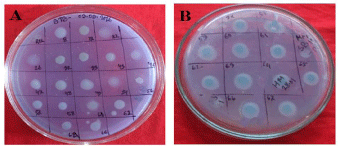
Figure 2: Isolated root nodulating rhizobial response to BTB test.
A: Isolated Mimosa Root Nodulating Strains, With Some Water Mimosa
Nodulating Strains; B: Isolated Water Mimosa Root Nodulating Strains.
Survival capacity of mimosa root nodulating rhizobia at acid-alkaline conditions
Soil acidity or alkalinity (extreme low or high pH) is another abiotic stress that affects legume and rhizobia growth and inhibits root nodule formation [22]. Though liming on acid soils has been followed as a common practice to raise the soil pH for creating a favorable conditions for the growth and survival of root nodule bacteria, it bears cost and farmers are not interested to use it [35]. Thus, best way to solve it is to identify acid insensitive strains for acidic soil and base insensitive strains for basic soils.
Recent evidence suggested high abundance of Burkholderia in acidic soils and different rhizobia could survive at pH 4.5-7.0 [18,20]. In our study, we categorized both acid and base tolerable strains via pH tolerance test. All isolated mimosa root symbionts/rhizobia were grew at pH 5.0 (Table 2) except the strain MB-08. Out of 13 mimosa strains, 5 strains (MB-43, MB-47, MB-48, MB-49, and MB- 56) grew weakly at pH 4 while 3 strains grew (MB-17, MB-32 and MB-33) moderately at highly acidic pH 4.0 (Table 3). At basic pH 9.0, 4 mimosa nodulating strains (MB-08, MB-26, MM-46 and MM-54) grew well; 5 strains viz. MB-17, MB-22, MB-33, MB-43 and MB-49 grew moderately; other 3 strains (MB-32, MB-47 and MB-48) grew slightly (Table 3). On the other hand, out of 13 mimosa strains, 6 strains viz. MB-08, MB-22, MB-26, MB-43, MM-46 and MM-54 grew moderately; 6 strains (MB-17, MB-32, MB-33, MB-47 and MB-49) grew slightly at pH 10.0 (Table 3). Though rhizobia from Neptunia (L. portucalens) is able to grow at pH range 4.0 but not able to survive at pH-10 [5]. All water mimosa root nodulating strains viz. MB-57, MB- 58, MB-59, MB-60, MB-61, MB-62, MB-63, MB-64, MB-65, MB-66 and MB-67 grew slightly at pH 5.0 (Figure 4) but none of them were availed to survive at pH-4; where all strains grew well at basic pH up to 10.0 (Table 3). Almost similar results also observed by authors [7] who reported that water mimosa root symbionts, A. Undicola grew well at pH of 4.0-10.0 though optimal growth was at pH 7.0-8.0. In general, water mimosa nodulating rhizobial strains grew well at more alkaline condition whereas most of the mimosa strains preferred moderate to high acidic condition. Some mimosa strains (MB-17, MB-32, MB-33, MB-43, MB-47, MB-48 and MB-49) survived at a wide range of pH (=4.0 to =10.0). We found few acid tolerant strains (MB-17, MB-32 and MB-33) which could be used in acidic soil and moderately base tolerant strains, MB-08, MB-22, MB-26, MB-43 and MM-54 could be used in basic soil with respective legume hosts for better growth. In comparison between mimosa and water mimosa, it is clear that strains of water mimosa are less acid tolerant than that of mimosa.
Isolate/strain no.
Avg. colony size (mm)
Colony shape
Colony color
BTB test
Colony Color
Growth
MB-08
3.2
R, C, S, M
M
Light blue
+++
MB-17
3.1
R, C, S, M
T
Green
++
MB-22
3.2
R, C, S, M
T
Light blue
+++
MB-26
3.3
R, C, S, M
T
Light blue
+++
MB-32
2.5
R, C, S, M
M
Green
++
MB-33
2.8
R, C, S, M
M
Green
++
MB-43
3.2
R, C, S, M
Mw
Green
++
MB-46
3.4
R, C, S, M
T
Light blue
++
MB-47
3
R, C, S, M
Mw
Green
++
MB-48
3.2
R, C, S, M
Mw
Green
++
MB-49
3.2
R, C, S, M
Mw
Green
++
MM-54
3.5
R, C, S, M
T
Light blue
+++
MB-56
2.2
R, C, S, M
Mw
Green
++
MB-57
2.6
R, C, S, M
Mw
Deep blue
+++
MB-58
2.8
R, C, S, M
Mw
Deep blue
+++
MB-59
2.7
R, C, S, M
Mw
Deep blue
+++
MB-60
2.3
R, C, S, M
Mw
Deep blue
+++
MB-61
2.9
R, C, S, M
Mw
Deep blue
+++
MB-62
2.3
R, C, S, M
Mw
Deep blue
+++
MB-63
2.8
R, C, S, M
Mw
Deep blue
+++
MB-64
1.8
R, C, S, M
Mw
Deep blue
+++
MB-65
1.9
R, C, S, M
Mw
Deep blue
+++
MB-66
1.9
R, C, S, M
Mw
Deep blue
+++
MB-67
2.5
R, C, S, M
Mw
Deep blue
+++
Abbreviations: C: Convex; M: Moderately Mucoid; Mw: Milky White in Color; R: Round Shaped; S: Smooth Edge; T: Transparent, ++: Moderate Growth; +++: Vigorous Growth
Table 2: Colony morphology of the isolated mimosa root symbionts and their response to BTB test.
Isolate/strain no.
NaCl (%)
pH
0.5-1.0
1.5-2.0
2.5
3
pH4
pH5
pH9
pH10
MB-08
++
-
-
-
-
-
+++
++
MB-17
+++
++
+
-
++
+++
++
+
MB-22
+++
++
+
-
-
+
++
++
MB-26
+++
++
+
-
-
+
+++
++
MB-32
+++
++
+
-
++
+++
+
+
MB-33
+++
++
+
-
++
+++
++
+
MB-43
++
+
-
-
+
+++
++
+
MB-47
++
+
-
-
+
+++
+
+
MB-48
++
+
-
-
+
++
+
++
MB-49
++
+
-
-
+
+++
++
+
MM-54
+++
-
+
-
-
+
+++
++
MB-56
+
-
-
-
+
+
-
-
MB-57
++
-
-
-
-
+
+++
+++
MB-58
++
-
-
-
-
+
+++
+++
MB-59
++
-
-
-
-
+
+++
+++
MB-60
++
-
-
-
-
+
+++
+++
MB-61
++
-
-
-
-
+
+++
+++
MB-62
++
-
-
-
-
+
+++
+++
MB-63
++
-
-
-
-
+
+++
+++
MB-64
++
-
-
-
-
+
+++
+++
MB-65
++
-
-
-
-
+
+++
+++
MB-66
++
-
-
-
-
+
+++
+++
MB-67
++
+
-
-
-
+
+++
+++
Abbreviations: +++: Well Grown, + +: Moderately Grown, +: Slightly Grown, -: Could Not Survived.
Table 3: Isolated root nodulating rhizobial tolerance to different salt, pH and temperature stress.

Figure 4: Isolated root nodulating rhizobial tolerance to different pH stress.
Mimosa nodulating rhizobial growth (left); water mimosa nodulating rhizobial
growth (right) at pH 4.0

Figure 1: Isolated root nodulating rhizobial growth at different temperatures.
D: Water Mimosa Nodulating Rhizobial Growth at 340C; G: Mimosa Nodulating
Rhizobial Growth at 430C.
Characterization of isolated root nodulating rhizobia based on salt tolerance test
Salinity is a major constrains for crop production in the southern region of Bangladesh. It decreases the nutrition uptake of plants, particularly Phosphorus, due to their binding with Calcium ions in salt-stressed soils. Salinity inhibits bacterial growth and nodulation process in the legumes. But salt tolerant growth promoting rhizobacteria (PGPR) has promising effect to overcome this limitation [18]. In this study, we found that all strains grown on 1% NaCl; but 14 strains were unable to survive at 1.5-2.0% NaCl (Table 2). While six strains such as MB-17, MB-22, MB-26, MB-32, MB-33 and MM-54 were grown up to 2.5% NaCl (Figure 3). The minimum salt tolerance capacity of all isolates was 1% but few strains were able to survive with 2.5% NaCl (Table 3). Studies [3,12,14] reported different level (0.5-3%) of salt tolerance by root nodulating rhizobacteria. From our study, we found few strains tolerant to high NaCl-stress, such as MB-17, MB-22, MB-26, MB-32, MB-33 and MM-54 those could be used as growth promoting rhizobia for mimosa and water mimosa inoculation grown in salt stress areas of Bangladesh. In comparison between mimosa and water mimosa, it is important to note that strains of water mimosa are less salt tolerant than that of dry land mimosa.
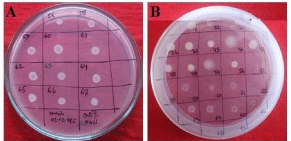
Figure 3: Isolated root nodulating rhizobial tolerance to different salt stress.
A: Growth of Water Mimosa Nodulating Strains at 0.5% Nacl; B: Mimosa and
Water Mimosa Nodulating Rhizobial Growth at 1.0% Nacl.
Temperature tolerance of isolated root nodulating rhizobia
Global climates change results in land degradation, salinity, desertification etc. which lead to reduction in nodule number, rhizobial growth, rate of colonization and infectious events, and can lead to delay in nodulation or restrict the nodule to the subsurface region [10]. But it has been reported that temperature tolerant soybean rhizobia can improve nitrogen fixation in areas where temperature is the major factor limiting production [35]. Isolates tolerant to temperature stress and with a high symbiotic effective were identified in the present work. Studies [1-4] reported the temperature tolerance of root nodulating rhizobia up to 420C. In our study, the isolated strains grew well at 31-340C. But in higher temperature they did not survive except four strains, MB-17, MB-32, MB-33 and MM-54 were survived up to 430C (Table 4). In another study [5] reported water mimosa root symbionts A. undicola grew well at temperatures ranging 25-350C and optimal growth were at 28-300C. Neptunia symbiont L. portucalensis, able to grow at temperature ranges 16-370C, where the optimum growth ranges 28-320C. In line with previous study, we found that water mimosa root symbionts grew up to 340C, but could not survive at 430C on YEMA plate up to 72 hours. For having high temperature tolerance potentialities of the strains, MB-17, MB-32, MB-33 and MM-54 could be used as growth promoting root nodules rhizobia of mimosa in the hot areas and could be important sources for temperature tolerant genes for biotechnological applications.
Isolate/strain no.
Temperature
10C
40C
310C
340C
370C
400C
430C
460C
MB-08
±
±
+++
+++
++
-
-
-
MB-17
±
±
+++
+++
+++
+++
+
-
MB-22
±
±
+++
+++
+++
+
-
-
MB-26
±
±
+++
+++
+++
+
-
-
MB-32
±
±
+++
+++
+++
+++
+
-
MB-33
±
±
+++
+++
+++
+++
+
-
MB-43
±
±
+++
+++
+++
+
-
-
MM-46
±
±
+++
+++
+++
++
-
-
MB-47
±
±
+++
+++
+++
+
-
-
MB-48
±
±
+++
+++
+++
+
-
-
MB-49
±
±
+++
+++
+++
+
-
-
MM-54
±
±
+++
+++
+++
++
+
-
MB-56
±
±
+++
+++
++
-
-
-
MB-57
±
±
+++
+++
++
-
-
-
MB-58
±
±
+++
+++
++
-
-
-
MB-59
±
±
+++
+++
++
-
-
-
MB-60
±
±
+++
+++
++
-
-
-
MB-61
±
±
+++
+++
++
-
-
-
MB-62
±
±
+++
+++
++
-
-
-
MB-63
±
±
+++
+++
++
-
-
-
MB-64
±
±
+++
+++
++
-
-
-
MB-65
±
±
+++
+++
++
-
-
-
MB-66
±
±
+++
+++
++
-
-
-
MB-67
±
±
+++
+++
++
-
-
-
Abbreviations: +++: Well Grown, + +: Moderately Grown, +: Slightly Grown, ±: Growth Not Clear, -: Could Not Grow
Table 4: Isolated root nodulating rhizobial tolerance to different temperatures.
Isolate/ strains no.
Plant weight (mg.)
Nodule nos.
Nodule weight (mg.)
MB-08
662f
34.0d
46.0a
MB-17
981.0c
38.0c
36.0c
MB-26
665.0f
17.0g
19.0e
MB-32
488.0g
26.0f
28.0d
MB-33
645.0f
13.0i
20.0e
MB-43
893.0d
35.0d
68.0a
MB-48
888.0d
31.0e
28.0d
MB-49
1690.0a
101.0a
67.0a
MM-54
1542.0b
72.0b
47.0b
Urea
475.0g
13.2h
15.4f
Control
422.0h
14.0h
18.0e
CV (%)
1.86
3.39
4.24
*Any two means having a common letter are not significantly different at 1% level of significance; ** indicates significant at 1% level of proximity.
Table 5: Effect of isolated rhizobial strains on mimosa growth.
Isolate/ strain no.
Plant weight (mg.)
Nodule nos.
Nodule weight (mg.)
MB-57
829.0e
24.0f
87.0d
MB-58
825.0e
23.0f
146.0a
MB-59
835.0e
24.0f
129.0b
MB-60
1325.0ab
45.0c
140.0a
MB-61
819.0e
57.6a
65.0f
MB-62
890.0d
27.8e
106.0c
MB-63
1098.0c
29.2e
125.0b
MB-64
589.0g
23.8f
130.0b
MB-65
1297.0b
32.4d
107.0c
MB-66
1360.0a
52.8b
105.0c
Urea
784.0f
12.8h
72.0ef
Control
742.0f
15.4g
79.0e
CV (%)
2.12
4.56
4.02
*Any two means having a common letter are not significantly different at 1% level of significance; **indicates significant at 1% level of proximity.
Table 6: Effect of isolated rhizobial strains on water mimosa growth.
Nodulation and growth of mimosa influenced by the inoculation with isolated rhizobial strains
Rhizobia stimulate the growth of host plants through N2 fixation. Studied strains were used for inoculation in pot experiment. We observed that, both number and weight of nodules influence the plant weight. Increased root nodule number and weight parallel increase the plant weight. After inoculation of selected mimosa nodulating strains (MB-08, MB-17, MB-26, MB-32, MB-33, MB-43, MB-48, MB-49 and MM-54) to mimosa plants, the mean dry weights of the plants varied from 1690.00mg to 422.00mg. The MB-49 exhibited the highest plant weight and nodule number. Where the lower growth was observed by plants inoculated with MB-32; even though it exhibited better plant growth compared to control and urea treated groups. The nodule weight were also enhanced significantly (p‹0.01) and ranged from 68.80mg to 15.40mg. Higher nodule weight was noticed by the strain MB-43, where MB-49 exhibits statistically similar effect on nodule weight; where lowest nodule weight was showed by plants inoculated with MB-26; but it exhibited better growth compared to control and urea treated groups (Table 5).
Similarly, after inoculation of 10 randomly selected water mimosa nodulating strains to its respective hosts, the highest nodulation was exhibited by the plant inoculated with MB-61; and the lowest inoculation was shown by urea treated groups. After inoculation of isolated strains, nodule numbers were significantly (p‹0.01) enhanced and ranged from 57.60 to 12.80. On the other hand, the highest nodule weight was noticed by MB-58, the effect of MB-66 on nodule weight was statistically similar to MB-58; where the lowest nodule weight was observed by plants inoculated with MB-61 (Table 6). The mean plant weight differed from 1360 to 589 mg. MB-66 exhibited the highest plant dry weight and the highest nodule number; where the lowest plant growth was exhibited by plants inoculated with MB-64; and it also exhibits lower growth compared to control and urea treated groups. The nodule number, nodule dry weight and plant dry weights were significantly increased after rhizobial inoculation. Similar to this result, other study [2] reported Thiobacillus sp. significantly enhanced the groundnut root, root length and plant biomass in pot and field experiments by 5-10%.
In comparison between mimosa and water mimosa, mimosa has lesser and smaller root nodule than that of water mimosa (Table 5 and Table 6). Likely, nodules produced by water mimosa strains are bigger than that of mimosa. But we can conclude that, the MB-49 strain is responsible for the highest plant dry weight and the height nodule number in mimosa (Table 5) and MB-66 strain in water mimosa showed statistically similar effect to highest nodule weight producing strains (Table 6). These two strains may be used as biofertilizer in future.
Mimosa and water mimosa play a significant role in sustainability of agricultural and environmental control systems and heavy metals accumulation; these plants also used as good sources of novel phytochemicals which are also influenced by their symbiotic rhizobia. So far, this is the first study of root nodulating rhizobia from mimosa and water mimosa from Bangladesh. Isolated rhizobial strains showed high diversity with different tests and also showed important characteristics such as capacity to tolerate at high concentration of salt, high temperature and highly acidic pH. It was found that plant dry weight, nodule number and nodule weight were increased when plants inoculated with isolated strains. We found some strains (MB-43 and MB-49) influences better growth and development for mimosa; where some other strains (MB-58, MB-60 and MB-66) exhibits better growth and development of water mimosa. Thus, the strains MB-49 and MB-58 could be used as bio-fertilizer for mimosa and water mimosa, respectively. Present study found that rhizobia from mimosa are very specific for nodule formation with their original host but two strains from water mimosa were availed to form nodules with three different type of mimosa. Present study on isolation and characterization of rhizobia from mimosa and water mimosa will extend their application in their production, biotechnological application, and agriculture practices and alleviation of salt stress affected soils.
References
- Alshaharani TS, Shetta ND. Phenotypic and biochemical characterization of root nodule bacteria naturally associated with woody tree legumes in Saudi Arabia. J Environ Biol. 2015; 36: 363-370.
- Anandham R, Sridar R, Nalayini P, Poonguzhali S, Madhaiyan M, Tongmin SA. Potential for plant growth promotion in groundnut (Arachis hypogaea L.) cv. ALR-2 by co-inoculation of sulfur-oxidizing bacteria and Rhizobium. Microbiol Res. 2007; 162: 139-153.
- Berrada H, Nouioui I, Houssaini MI, Ghachtouli NEL, Gtari M, Benbrahim KF. Phenotypic and genotypic characterizations of rhizobia isolated from root nodules of multiple legume species native of Fez, Morocco. Afr. J. Microbiol. Res. 2012; 6: 5314-5324.
- Bhunia D, Mondal AK. Systematic Analysis (Morphology, Anatomy and Palynology) of an Aquatic Medicinal Plant Water Mimosa (Neptunia Oleracea Lour.). In Eastern India. Int. j. life sci. biotechnol. pharma res. 2012; 1: 290- 319.
- Carvalho MF, Marco PD, Duque AF, Pacheco CC, Janssen DB, Castro PML. Labrys portucalensis sp. nov., a fluoro benzene degrading bacterium isolated from an industrially contaminated sediment in northern Portugal. Int J Syst Evol Microbiol. 2008; 58: 692-698.
- Chen LS, Figueredo A, Michajluk HVJ, Hungria M. Diversity and symbiotic effectiveness of rhizobia isolated from field-grown soybean nodules in Paraguay. Biol Fertil Soils. 2002; 35: 448-457.
- Chen WM, de Faria SM, Chou JH, James EK, Elliott GN, Sprent JI. Burkholderia sabiae sp nov., isolated from root nodules of Mimosa caesalpiniifolia. Int J Syst Evol Microbiol. 2008; 58: 2174-217
- Gauri Singh AK, Bhatt RP, Pant S, Bedi MK, Naglot A. Characterization of Rhizobium isolated from root nodules of Trifolium alexandrinum. J. Agr. Tech. 2011; 7: 1705-1723.
- Gomez KA, GomezAA. Statistical procedures for Agricultural Research. 2nd edition. Jhon Wiely and Sons. New York. 1984; 207-215.
- Gopalakrishnan S, Sathya A, Vijayabharati, R. 3 Biotech 5. 2015. pp. 355- 377. Jamuna DY, Devi SP, Singh HT. Antiimplantation and antiestrogenic activity of the leaf extract of Mimosa pudica L. in female albino rats. Indian Drugs. 2001; 38: 414-417.
- Kimata N, Nishino T, Suzuki S, Kogure K. Pseudomonas aeruginosa isolated from marine environments in Tokyo Bay. Microb Ecol. 2004; 47: 41–47.
- Laguerre G, Depret G, Bourion V, Duc G. Rhizobium leguminosarum bv. viciae genotypes interact with pea plants in developmental responses of nodules, roots and shoots. New Phytol. 2007; 176: 680-690.
- Mahenthiralingam E, Baldwin A, Drevinek P, Vanlaere E, Vandamme P. Multilocus Sequence Typing Breathes Life into a Microbial Metagenome. PLOS ONE. 2006.
- Mendez MO and Maier RM. Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Perspect. 2008; 116: 278-283.
- Michael BJ, Emeritus R, Dale HR, Seth LN, Bennett NB. The Minnesota Soybean Field Book. St Paul MN: University of Minnesota Extension. 2014; 79.
- Muthumani R, Meera P, Devi LV, Seshu KK, Sivaram M Badmanaban R. Phytochemical Investigation and Enzyme Inhibitory Activity of Mimosa pudica Linn. Journal of Chemistry and Pharmacology Research. 2010; 2: 108-114.
- Nogales B, Moore ERB, Llobet-Brossa E, Rossello-Mora R, Amann R. Timmis KN. Combined Use of 16S Ribosomal DNA and 16S rRNA To Study the Bacterial Community of Polychlorinated Biphenyl-Polluted Soil. Appl. Environ. Microbiol. 2001; 67: 1874-1884.
- Pinedo I, Ledger T, Greve M, Poupin MJ. Burkholderia phytofirmans Ps JN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front Plant Science. 2015; 6: 466.
- Rajendran R, Krishna KE. Hypolipidemic Activity of Chloroform Extract of Mimosa pudica Leaves. Avicenna J Med Biotechnol. 2010; 2: 215-221.
- Reis VM, Estrada-de los SP, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, et al. Burkholderia tropica sp. Nov., a novel nitrogen-fixing, plantassociated bacterium. Int J Syst Evol Microbiol. 2004; 54: 2155-2162.
- Richardson AE, Djorjevic MA, Rolfe BG, Simpson RJ. Effect of pH, Ca and Al on the exudation from clover seedlings of compounds that induced the expression of nodulating genes in Rhizobium trifolii. Plant and Soil. 1988; 109: 34-47.
- Rusch A, Islam S, Savalia P, Amend JP. Burkholderia insulsa sp. nov., a facultatively chemolithotrophic bacterium isolated from an arsenic-rich shallow marine hydrothermal system. Int J Syst Evol Microbiol. 2015; 65: 189-194.
- Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth promoting bacterial endophyte. Appl Soil Ecol. 2012; 61: 217-224.
- Sadowsky MJ, Keyser HH, Benbohlool B. Biochemical characterization of fast and slow-growing rhizobia that nodulate soybeans. Int J Syst Evol Microbiol. 1983; 33: 716-722.
- Somasegaran P, Hoben HJ. Handbook for rhizobia: Methods in legumerhizobium technology. Springer, Berlin Heidelberg New York. 1994.
- Syuhaida AW, Sharifah NSI, Emilia ZA, SarvaMangala P. Neptuniaoleracea (water mimosa) as phytoremediation plant and the risk to human health: A review. Adv. Environ. Biol. 2014; 8: 187-194.
- Taghavi S, Mergeay M, Nies D, van der Lelie D. Alcaligenes eutrophus as a model system for bacterial interactions with heavy metals in the environment. Res. Microbiol. 1997; 148: 536-551.
- Tan KZ, Radziah O, Halimi S, Khairuddin AR, Habib SH and Shamsuddin ZH. Isolation and characterization of rhizobia and plant growth-promoting rhizobacteria and their effects on growth of rice seedlings. Am. J. Agri. and Biol. Sci. 2014; 9: 342-360.
- Towe K. Evolution of nitrogen fixation. Science. 2002; 295: 798-799.
- Valsala S, Karpagaganapathy PR. Effect of Mimosa pudica root powder on oestrous cycle and ovulation in cycling female albino rat Rattus norvegicus. Phytotherapy Res. 2002; 16: 190-192.
- Valsala S. Estrogenic and antiestrogenic activities of Mimosa pudica on Rattus norvegicus. J of Ecotoxicology. 2000; 10: 25-29.
- Veschasit O, Meksumpun S, Meksumpun C. Heavy Metals Contamination in Water and Aquatic Plants in the Tha Chin River, Thailand. Kaset J (Natu Sci). 2012; 46: 931-943.
- Wang X, Wu N, Guo J, Chu X, Tian J, Yao B, Fan Y. Phytodegradation of organ phosphorus compounds by transgenic plants expressing a bacterial organ phosphorus hydrolase. Biochemical and Biophysical Res. Com. 2008; 365: 454-458.
- Watkin ELJ, O’Hara GW, Glenn AR. Calcium and acid stress interact to affect the growth of Rhizobium leguminosarum bv. trifolii. Soil Biol Biochem. 1997; 29: 1427-1432.
- Zhang H, Prithiviraj B, Charles TC, Driscoll BT, Smith DL. Low temperature tolerant Bradyrhizobium japonicum strains allowing improved nodulation and nitrogen fixation of soybean in a short season (cool spring) area. European J of Agr. 2003; 19: 205-213.