
Research Article
Bacteriol Mycol. 2018; 5(2): 1067.
Ramie (Boehmeria nivea) Gum: A Natural Feed to Sustain and Stimulate the Growth of Bacteria
Banerjee S1, Gupta M2, Roy A2, Chakraborty A1 and Ray Chaudhuri S1*
¹Department of Microbiology, Tripura University, Suryamaninagar, India
²Centre of Excellence in Environmental Technology and Management, Maulana AbulKalam Azad University of Technology, West Bengal, India
*Corresponding author: Shaon Ray Chaudhuri, Department of Microbiology, Tripura University, Suryamaninagar, India
Received: April 06, 2018; Accepted: May 03, 2018; Published: May 10, 2018
Abstract
Bacteria require nutrients to sustain growth. For most of the commonly used complex media, the components are obtained from animal slaughter and are expensive. They support growth but when discharged, lead to environmental pollution and sustain growth of undesirable microbes. Thus it is necessary to look for cheaper and less enriched alternative sources for bacterial nutrition. Gum (1% w/v) from the decorticated fibers of Boehmeria nivea (Ramie), supported growth to different extent for both Bacillus sp and Pseudomonas sp. The growth was further enhanced with addition of Tryptone, Yeast Extract and sodium chloride (Luria Bertini broth) to the gum at a ratio of 1:3 for bacteria of both Genus Bacillus and Pseudomonas. This could be an alternative bacterial growth option for activities resulting in environmental discharge of the effluent like fiber degumming, waste water treatment, biofertilizer production, to name a few.
Keywords: Ramie; Gum; Bacterial Feed; Growth
Introduction
All living organisms require food to grow, reproduce and carry out their normal metabolic functions, and so does bacteria. They usually utilize organic matter to derive energy. The dependence is more on simpler substances with low molecular weight which can be easily broken down by extracellular enzymes [1]. They can live on a range of substances ranging from starch, milk, wood and even sulphur. Some thrive on substances such as body fluids in which they live [9].
For the cultivation of bacteria in the laboratory, two types of complex media, Nutrient broth and Luria Bertani (LB) broth are routinely used. They are composed of substances like beef extract, peptone, tryptone which are obtained from animal slaughter, making them costly and not environment friendly during discharge. Thus it would be beneficial if the use of such materials could be reduced if not replaced with products from plant origin. It would in turn reduce grain and water consumption used for animal feeding while reduces environmental pollution due to discharge of used medium following large scale cultivation.
Many plants like Boehmeria nivea (Ramie) produce gum as a secondary metabolite [11] resulting from cellulose disintegration [5] and is often unwanted for the plant themselves as well as the plant derived products like fiber for fabric production. For textile industry, the Ramie bast fiber, after the removal of gum by natural or chemical means is used for the production of fabrics which is often blended with other fibers such as cotton [8]. During the process of natural retting, the fibers dipped in water swell due to water penetration where the outer layers absorb moisture and become more exposed to decay causing bacteria. In this process the gum from the bastfibers oozes out into the water in which it remains dipped for a standard period of time [10]. The Ramie gum composed of cellulose, hemicellulose, lignin and pectin is a rich carbon source [3] which is usually discarded after degumming, contaminating the environment. These gum constituents are reported to be utilized by bacteria [7,12,2] provided they would break down these constituents into simpler form. With this background knowledge we attempted to utilize the Ramie gum as a feed for bacteria with an objective to minimize cost as well as environmental damages.
Materials and Methods
Standardization of natural retting at laboratory scale
Decorticated Ramie fibers obtained from Indian Institute of Technology, Kharagpur (IITKGP), West Bengal, India were dipped in tap water and the process of natural degumming was standardized 6. The gum was extracted from the liquor component [4].
Gum as microbial feed
The extracted gum was used at a concentration of 1% (w/v) in water to prepare liquid medium for bacterial growth. Two sets of medium were prepared: One was sterilized by filtration through 0.45μm filter units inside a laminar flow hood, and the other was sterilized at 15 psi for 15 minutes. Each set was separately inoculated with Bacillus sp (SRC 005, SRC 007, MCC2138, MTCC 11281, MCC2071, MCC2059, MCC0008) and Pseudomonas sp (SRC 001, MTCC 5564 and SRC 004) followed by incubation for 16 hours at 37°C and shaking instead of 200 rpm (SI - 300 Lab Companion). Growth in terms of the optical density at 600nm (Eppendorf bio Spectrophotometer) was measured and compared among the sets to check for the presence of thermo labile components in the gum.
Media containing different ratios (1:3, 1:1, 3:1) of gum with LB were prepared to check the impact of the combination on microbial growth. As control different percentages of LB (25%, 50%, 75% and 100%) were used. LB at 100% concentration consisted of 1% Tryptone, 0.5% sodium chloride and 0.5% Yeast Extract. Each media preparation was sterilized by autoclaving at 15 psi for 15 minutes.
Seven different strains of Bacillus sp and three different strains of Pseudomonas sp, as mentioned above, were inoculated (1% v/v) and incubated overnight. The optical density was measured to determine the optimum combination to support bacterial growth using gum.
Amount of gum extracted from identical weight of Ramie fiber is an indication of the amount of gum produced in response to any treatment. The impact of fertilizer [chemical (NPK), vermicompost (generated using dried Ramie cortex and leaves), and liquid biofertilizer] application on the gum production in Ramie was assessed using standard method [6].
Statistical validation
All of the statistical tests were performed using Microsoft Excel 2007. A t-test was conducted at the 5% level of significance to evaluate the validity of the results.
Results and Discussion
The gum extraction indicated maximum degumming within 72 hours at 40°C (room temperature in June 2015) with a fiber: Liquor ratio of 1:8 [6].
The various isolates displayed different extents of growth in autoclaved and filter sterile gum solution. Some isolates (MCC2138, MCC2059, SRC 005, SRC 007 and SRC 004) exhibited better growth in filter-sterilized gum, indicating requirement of thermolabile gum components that might have degraded during autoclaving. The remaining isolates grew well in autoclaved gum, which indicated that the thermolabile components in the gum solution had an inhibitory effect on their growth (Figure 1). While pectine degrades at elevated temperature (45o to 58oC), hemicellulose degrades at elevated pressure. These could be responsible for the variation in bacterial growth with and without autoclaving the gum solution.
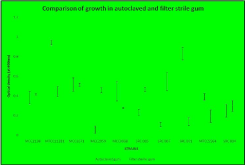
Figure 1: Comparison of the growth of different isolates in the presence of
autoclaved and filter-sterilized gum solution.
The growth of the above mentioned ten isolates in different combinations of gum and LB medium is shown in Figure 2.
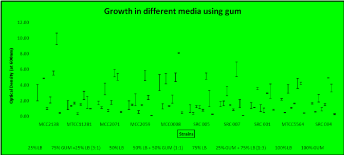
Figure 2: Growth of isolates in different combinations of gum and LB medium.
All the isolates displayed an enhancement of growth in the presence of gum: LB ratio of 1:3 as compared to LB alone in the same concentration (75%) indicating some essential bacterial growth stimulators in gum for both Bacillus sp and Pseudomonas sp. The fold enhancement in each case is shown in Table 1.
STRAIN
Fold enhancement
P VALUE
MCC2138
2.7
0.003
MTCC 11281
1.8
0.03
MCC2071
3.14
0.01
MCC2059
3.7
0.01
MCC0008
2.3
3.55 E-05
SRC 005
6.7
0.002
SRC 007
7.3
0.01
SRC 001
3.8
0.003
MTCC 5564
2.6
0.014
SRC 004
3.3
0.00042
Table 1: The fold enhancement in growth in 25% gum with 75% LB using 75% LB as the control. Here, 100% LB is composed of 1% tryptone, 0.5% yeast extract and 0.5% sodium chloride. Growth enhancement in each case is significant.
However, when the proportions were reversed (gum: LB ratio of 3:1), no significant growth enhancement was observed as compared to same concentration of LB (25%) indicating requirement of higher concentration of nutrients or additional nutrients (other than gum) for optimum growth of microbes. The gum could be only utilized as a carbon source by the bacteria during periods of restricted supply of enriched media. Based on our findings, it is evident that Pseudomonas sp (89.1%) and Bacillus sp (85.6%) can utilize similar amounts of gum to sustain their growth in gum-containing water. The gum defusing from the fiber sap into the soaked water functions as growth medium for bacteria. The ability of the bacteria to utilize the gum depends on their enzyme-producing ability, which in turn degrades the gum to an utilizable form.
Rhizome planted once produces shoot for the next twenty years where the fiber can be harvested 3 to 6 times a year. In order to ensure maintained production, fertilizer application is a must. The effect of fertilizer (chemical, vermicompost as well as liquid bioformulation) on gum production as compared to control (without fertilizer) revealed significant enhancement of gum production when treated with chemical fertilizer (P value was 0.001); vermicompost of Ramie origin (P value was 0.01) as well as liquid biofertilizer (P value was 0.02). Hence fiber yield enhancement is associated with enhancement of gum yield.
Conclusion
Use of low concentrations of gum along with Luria Bertani medium could be a better alternative that is cheaper and more eco friendly. The combination of gum and LB in a 1:3 ratio could act as a growth stimulant. Through this approach what is a waste in Ramie cultivation (dried leaf, stock, plant sap) can be converted into byproducts. The byproduct includes the vermicompost that in turn could enhance production of the other byproduct (bacterial feed, Gum).
Acknowledgements
The authors would like to acknowledge the financial assistance of the Ministry of Human Resource Development (MHRD), Government of India (GOI) under the Frontier Area of Science and Technology scheme for conducting the work and providing studentship to M Gupta; University Grants Commission under the UGC - DAE - CSR scheme for providing the fellowship to S. Banerjee; Maulana AbulKalam Azad University of Technology, West Bengal (formerly known as West Bengal University of Technology, India) as well as Tripura University for providing the laboratory space and computational facility.
References
- Allison SD, Vitousek PM. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil. Biol. Biochem. 2005; 37: 937-944.
- Banik S, Ghosh SN. Pectinolytic activity of microorganisms in piling of jute. Indian Journal of Fiber and Textile Research. 2008; 33: 151-156.
- Bast Fibres: Ramie. 2017.
- Duan S, Liu Z, Feng X, Zheng K, Cheng L, Zheng X. Diversity and characterization of ramie-degumming strains. Sci. Agric. 2012; 69: 119-125.
- Gums and Resins. 2017.
- Gupta M, Roy A, Banerjee S, Kapoor R, Adhikari B, Thakur AR, et al. Bacillus sp MCC2138: A potential candidate for microbial degumming of Ramie. International Journal of Fiber and Textile Research. 2015; 5: 39-43.
- Gupta P, Samant K, Sahu A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012.
- Kalita BB, Gogoi N, Kalita S. Properties of ramie and its blends. Int. J. Eng. Res. Gen. Sci. 2013; 1: 1-6.
- Parke D, D’Argenio DA, Ornston LN. Bacteria are not what they eat: That is why they are so diverse. J. Bacteriol. 2000; 182: 257-263.
- Retting. 2017.
- Wink M. Introduction: Biochemistry, physiology and ecological functions of secondary metabolites. In: Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism (Wink M, ed). Oxford, UK: Wiley-Blackwell. 2010: 1-19.
- Ventorino V, Aliberti A, Faraco V, Robertiello A, Giacobbe S, Ercolini D, et al. Exploring the microbiota dynamics related to vegetable biomasses degradation and study of lignocellulose-degrading bacteria for industrial biotechnological application. Sci. Rep. 2015; 5: 8161.