
Research Article
J Bacteriol Mycol. 2018; 5(3): 1068.
Inhibition of Biofilm Formation in Candida albicans and Candida krusei by Combretum zeyheri Leaf Extracts
Mtisi B1, Sithole S2, Mombeshora M2 and Mukanganyama S2*
¹School of Pharmacy, University of Zimbabwe, Mt. Pleasant, Harare, Zimbabwe
²Department of Biochemistry, University of Zimbabwe, Mt. Pleasant, Harare, Zimbabwe
*Corresponding author: Stanley Mukanganyama, Biomolecular Interactions Analyses Group, Department of Biochemistry, and University of Zimbabwe, Zimbabwe
Received: April 13, 2018; Accepted: May 16, 2018; Published: May 23, 2018
Abstract
Fungal infections are currently on the increase due to use of invasive devices such as indwelling catheters, antibiotic misuse and HIV/AIDS infections. Antifungal drug resistance may be due to biofilm formation, polymorphism or phenotypic switching. Combretum zeyheri is used for the treatment of various ailments such as snakebite, diarrhea, hypertension and eye infections. The aim of this study was to identify C. zeyherileaf extracts that inhibit biofilm formation in Candida species. Eight extracts were screened to for antifungal activity using broth micro dilution assay. The screening for inhibition of biofilm by the extracts was determined using biofilms formed on 1cm2 PVC square disks. The amount of biofilm formed was determined spectrophotometrically after staining with crystal violet. The ethanol extract, ethyl acetate extract, acetone extract showed potent antifungal activity against C. albicans whilst in C. krusei, it was the methanol, ethanol, ethyl acetate and hexane extracts. The methanol extract, hexane extract, DCM extract and DCM-methanol extract showed potent inhibition of biofilm formation in C. albicans whilst in C. krusei only the water extract and ethanol extract showed inhibition of biofilm formation. The DCM extract was the most effective in inhibition of biofilms. The minimum inhibitory concentration for biofilm formation for the DCM extract was 62.5μg/ml in C. krusei and 50μg/ml in C. albicans. In addition to inhibition of growth; C. zeyheri extracts inhibit biofilm formation in both C. albicans and C. krusei. The isolation of active components that exhibit both antifungal and anti-biofilm formation from C. zeyheri is required.
Keywords: Combretum zeyheri; Candida albicans; Candida krusei; Biofilm Formation; Candidiasis
Introduction
The biggest challenge to all modern day clinicians is appearance of new or resistant strains of any disease causing microorganism [1]. Microbial resistance is on the rise and it is a cause for concern and a need arises for new pathways to be explored and to discover possible answers to nature’s new challenges which are presented through microbial evolution [2]. The use of herbal or plant remedies has been part of the primary health care system in Zimbabwe for a long time and they are now commonly integrated as complements to conventional medicines [3]. Literature documents claims about the antimicrobial properties of many plants all over the world [4]. The Combretaceae family is one of the plant species extensively explored with a wide array of antimicrobial activity and plays a large part in African ethno medicine [5].
The candida genus is a heterogeneous group microorganism and 17 different species are known to cause human infections. Candida albicans, Candida krusei, Candida parapsilosis and Candida glabrata are the common species which result in over 90% of all invasive infections [6]. C. albicans is a commensal microorganism found on human skin, mucosal oral cavity, gastrointestinal tract and vagina [7]. Its presence in a healthy individual does not cause any problems, but this may change however in immune-compromised individuals. Virulence factors are genetically determined, but expressed by microorganisms when subjected to certain conditions [8]. Virulence characteristics specialized and developed by Candida spp. are involved in invasion and adhesion of host tissues, biofilm formation and evasion of the immune system and the infection [9]. Virulence factors are expressed when a suitable environment persists and these include polymorphism [10], production of adhesins and invasins [11].
Biofilms are communities found within a matrix of extracellular material. The matrix is a three–dimensional, gel-like hydrated and charged environment in which the microorganisms are immobilised [12]. Within a matrix there are channels that provide a mechanism for nutrient circulation. Failure by antimicrobial agents to penetrate is due to the biofilm matrix. The drugs that by-pass are assisted by water channels within the biofilm. The microbial biofilm matrix components could also retard access to drug via adsorption or neutralisation [13]. In general, the biofilm matrix comprises of carbohydrates, proteins, phosphorus and hexosamines. Environmental conditions such as medium composition, pH and oxygen as well as the fungal strain and species affect biofilm formation and matrix composition [14]. Biofilms can grow on any biotic or a biotic moist surface. Formation of biofilms by microorganism is also a defense mechanism for their own survival in a hostile environment [15].
Plants have been shown to contain more compounds than chemists can synthesize and have served as a source of new drugs, pharmaceutical products and provide starting materials for synthesis of many known drugs [16]. There are at least twenty four known species of Combretum family commonly used in African traditional medicine and these form the basis of most traditional health care in the management of a variety of ailments and diseases [17]. Traditionally Combretum zeyheri is used for the treatment of various ailments such as snake bite, diarrhea and hypertension. In Zimbabwe, the plant is used for diarrhea and eye infections [18]. Combretaceae and Terminalia spp have been used for the management of fevers, cough, respiratory infections, urinary tract infections, piles and worms and also useful in treating chronic diarrhea and dysentery, flatulence, vomiting, colic and enlarged spleen and liver [19].
For in vitro studies using antibacterial agents, susceptibility is commonly quantified using minimum inhibitory concentrations (MICs) to determine the lowest concentration required to exert growth inhibition. Extracts of C. zeyheri have been reported to have antimicrobial activity C. albicans, C. krusei and C. parapsilosis [20]. Isolated phytochemicals showed antifungal activity on laboratory strains and the clinical fungal isolates [21]. In a study by Mangoyi et al, [22], C. zeyheri extracts showed to be effective antifungal and drug efflux inhibitors. Mutasa et al [23], showed that C. zeyheri ethanolic extract produced a dose-dependent effect on ergosterol synthesis in C. albicans by disrupting membrane integrity. Mangoyi, et al, [24] isolated and from C. zeyheri that showed antifungal activity in both C. krusei and C. albicans. The flavonoids also showed effective inhibition of ergosterol synthesis in C. albicans. From these studies, C. zeyheri extracts has established antifungal properties, but there are few studies on its effects on virulence factors such as the ability to affect biofilm formation. The aim of this study was to determine the effects C. zeyheri extracts on biofilm formation in C. albicans and C. krusei.
Methods and Materials
Fungal strains, reagents
The following reagents and chemicals used were obtained from Sigma-Aldrich (Taufkirchen, Germany): MTT (3-(4, 5-dimethylthiazol-2-yl_-2, 5-diphenyltetrazolium bromide), DMSO, miconazole, Sabouraud’s dextrose broth (SDB), Sabouraud’s dextrose agar (SDA), crystal violet, and barium chloride. C. albicans ATCC 10231 and Candida krusei clinical strain was obtained from Parirenyatwa Group of Hospitals, Harare, Zimbabwe.
Plant collection
Leaves of C. zeyheri were collected from Norton (170 53' 0'' S, 300 42' 0'' E) Mashonaland West Province of Zimbabwe. The plant identity was authenticated and classified by Mr. C. Chapano, a taxonomist at the National Herbarium and Botanical Gardens (Harare, Zimbabwe). The herbarium samples (voucher specimen N6E7) were kept in the Department of Biochemistry, University of Zimbabwe.
Preparation of plant extracts
Leaves were dried for five days in a Labcon orbital incubator (Labotec Co., Cape Town, South Africa) at 40°C. The leaves were ground by a manual / traditional pestle and mortar to a fine powder. C. zeyheri extract was prepared by weighing 50g of powder and adding 500ml of solvent. A mixture of 50:50 DCM: Methanol was used. The mixture was left to stir for 24 hours on a magnetic stirrer. After 24 hours to prepare the total extract the mixture was first prefiltered with cotton wool. The resultant filtrate was re-filtered with a Whatman™ no.1 into a pre-weighed plastic beaker. The resultant filtrate was then air dried under a fan. After drying the residue, the second solvent was added. This serial extraction was done using solvents in the following order of increasing polarity: Hexane, DCM, ethyl acetate, acetone, ethanol, methanol and water.
Growth of Candida species
C. albicans or C. krusei from glycerol stocks were resuscitated by subculturing in SDB for 24h at 370C in a Lab Companion incubator (Jeio Tech, Seoul, South Korea) shaking at 120rpm. The resulting inoculum was streaked on SDA plates and colonies from these plates were once more cultured in SDB. The optical density of C. albicans or C. krusei was adjusted with SDB to match that of 0.5 McFaland’s standard at 600nm. Appropriate dilutions were made to get a final inoculum concentration of 2×106cfu/ml.
Antifungal susceptibility tests using C. zeyheri leaf extracts
The effect of miconazole and the eight C. zeyheri leaf extracts obtained from the serial exhaustive and total extraction was investigated using the microbroth dilution method designed by Scorzoniet al, [26]. A stock solution of miconazole or the plant extracts were prepared by dissolving 0.005g in 1.25ml absolute DMSO and made up to 25ml by SDB media to a concentration of 200μg/ml. Two-fold serial dilutions were made to end up with concentrations of 100, 50, 25, 12.5, 6.3, 3.2, 1.6, 0.8 and 0 ug/ml. Aliquots of 100μl of solutions and 100μl of standardized cells culture were added to each well on a 96-well microplate. The negative control contained 0μg/ml of the extract and the antibiotic miconazole was the positive control. For the plant extracts the positive control included wells containing miconazole and cells as well as wells containing cells only. The final concentration of DMSO in each well was 2.5%. Absorbance of the wells was read at 590nm using a Genios Pro microplate reader (Tecan, Grödig, Austria) and the plate were incubated for 24h at 370C. After incubation absorbance of the plate was read again at 590nm in the ELISA reader to determine the change in cell density. MTT was added to determine cell viability. Cells were incubated for 2 hours after which 25μL of DMSO were added to dissolve the formazan crystals.
Screening of plant extracts for inhibition of biofilm formation by Candida species
A stock solution of extracts was prepared by dissolving 5mg of extract and dissolved in 1.25ml of absolute DMSO and made up to 25ml with SDB media. An overnight culture of cells was prepared. The cells were centrifuged in a Hettich Rotofix 32 (Tuttlingen, Germany) and washed once with 0.9% saline solution. The cells were standardized to a final count of 1.5 x108 cfu/ml. The method used for quantification of biofilm was a modification of one described by Borucki et al., [27]. Squares of 1cm x 1cm polyvinylchloride (PVC) were prepared and sterilized in 70% ethanol for overnight. Four disks were placed in each well of a six-well plate. An aliquot of 1.5ml of SDB media was added to each well and 1.5ml the standardized cell suspension was added to each well. The plates were incubated for 3h at 370C shaking at 80rpm in a Lab Doctor Mini Incubated Shaker (MidSci, California, USA). After 3h the cells disks were washed once with phosphate bicarbonate saline buffer. The disks were transferred into 12-well plates. Each well of the 12 well plates had 1.5ml aliquot of extract and 1.5ml of DMSO diluted in SDB media. Each extract was in duplicate, the third well was a control with extract, DMSO in media and a disk with no cells on it. The controls were disks in cells only, disks in cells + diluted DMSO, disks in miconazole at MIC and disks in media only. The plates were incubated at 370C shaking at 80rpm for 72h. Biofilm formation was determined in triplicate for each sample.
After 72h the well the free cells were decanted and the squares were washed three times with PBS. The wells were filled with 3ml 0.1% crystal violet and left to stand at room temperature for 45min. The crystal violet solution was removed and the wells and disks washed with PBS buffer five times. The plates were left to dry under a fan. After drying the crystal violet was dissolved in 95% ethanol, by adding 3 ml of the ethanol to each well. The crystal violet was left to solubilize for 30min. The dissolved crystal violet was transferred to 96-well plate. Each well from the 12-well plate contributed to 3 wells on the 96 well plates. The absorbance of crystal violet which was directly proportional to biofilm formation was read at 590nm in a Genios Pro ELISA reader (Tecan, Grödig, Austria).
Determination of MIC for the extract with most potent biofilm formation inhibiting capacity
A stock solution of 1000μg/ml was prepared by dissolving 25mg of extract in 1.25ml of DMSO and made up to 25ml with SDB media. Serial broth dilutions of the stock solution were made to 500, 250, 125, 62.5, 31.5, 15.8, 7.9 and 0 μg/ml. The disks of 1cm 2 were prepared and after incubating, the disks were placed in wells of the 12 well plates with 1.5ml aliquot of extract and 1.5ml of DMSO diluted in SDB media. Each concentration had a duplicate and a 3rd well was a control with extract, DMSO in media and a disk with no cells on it. The controls were disks in cells only, disks in cells + diluted DMSO, disks in miconazole at MIC and disks in media only. The plates were incubated at 370C shaking at 80rpm for 72 hours. After 72 hours, the extract suspensions were removed and the wells washed with PBS buffer three times. The wells were then filled with 0.1% crystal violet, 3ml each well and left to stand at room temperature for 45min. The crystal violet solution was removed and the wells and disks washed with PBS buffer five times. The plates were left to dry under a fan and ethanol was added to solubilize crystal violet after which absorbance of the dye was determined at 590nm.
The effects of combining potent anti-biofilm extract with a standard antifungal drug
A stock solution of 1000μg/ml was prepared by dissolving 25mg of extract in 1.25ml of DMSO and made up to 25ml with SDB media. Two-fold serial broth dilutions of the stock solution to give the following concentrations: 1000, 500 and 250 μg/ml. An overnight culture of cells was prepared. The cells were centrifuged in a Hettich Rotofix 32 (Tuttlingen, Germany), washed once with 0.9% saline solution and resuspended in PBS. The optical density of the cells were adjusted to McFarland standard to give a final count of 1.5 x108 cfu/ml. Biofilm formation was measured as described above but now including a combination of the extract and miconazole. A stock solution of 200μg/ml miconazole was prepared and diluted to concentrations of 25, 12.5 and 6.3 μg/ml for C. albicans and 12.5, 6.3 and 3.2 for C. krusei. A modification of checker board assay described by [28] was done. The square disks were transferred to 12 well plates. Each of the nine combinations was done in duplicate on the 12-well plate. Controls with combination concentrations and a disk with no cells were prepared for each combination. Other controls were miconazole only at MIC, cells with DMSO, media only and cells only. Each well contained 1.5ml of media with DMSO and 1.5ml of combination concentration in 50:50 ratio. The plates were incubated at 370C shaking at 80rpm for 72h in a Lab Doctor Mini Incubated Shaker (Mid Sci, USA). After 72h, the extract suspensions were washed off three times using PBS. The wells were then filled with 0.1% crystal violet, 3ml each well and left to stand at room temperature for 45min. The crystal violet solution was removed and the wells and disks washed with PBS buffer five times. The absorbance of the crystal violet from the disks was similarly determined as described above.
Statistical analyses
Statistically significant differences between the mean of the controls and the tests were analysed using one way ANOVA with Dunnett’s multiple comparison post-test using Graph Pad Prism 5 (Version 5.03 Graph Pad Software Inc. San Diego, California USA).
Results
Extraction of phytochemicals from C. zeyheri leaves
Solvent extraction is a method used to separate phytochemicals from plants. Solvents used separate them according to solubility in the solvent used or polarity. The yield is an indicator of the types of constituents in the leaves. After extraction and drying the water extract had the largest yield (12%), followed by DCM/Methanol (9%), ethanol (3.8%), acetone (3.7%), methanol (3.2%), hexane (2.3%), DCM (1.65%) and ethyl acetate extract (1.63%).
The effects of DMSO on cell viability
DMSO was the solvent of choice used to dissolve the extracts. All the extracts were soluble in DMSO. This assay was done to determine the possible toxic effects of the solvent to the fungal cells. C. albicans showed greater sensitivity to DMSO compared to C. krusei. Concentration of up to 5% DMSO had no effect on cell viability in C. krusei unlike in C. albicans where sensitivity began at concentrations above 2.5%.
Determination of MIC of Miconazole in C. albicans and C. krusei
Miconazole was used as the positive control in all the assays. The minimum inhibitory concentration was determined too as it was used as the reference standard. The results in showed that Candida krusei (MIC =3.2μg/ml) was more sensitive to the effects of miconazole when compared to C. albicans (MIC= 6.3μg/ml).
The effects of C. zeyheri leaf extracts on Candida growth
The ethanol extract was found to be the most effective at inhibiting the growth of C. albicans with an MIC of 3.2μg/ml, followed by methanol with an MIC of 25μg/ml (Figure 1). The ethyl acetate extract and acetone extract also had inhibitory activity, but MIC was higher than the highest concentration of 100μg/ml. The DCM-methanol extract showed no growth inhibitory activity in C. albicans for the range of concentrations used in the assay. For C. krusei the ethanol extract again had the most potent inhibitory activity with an MIC of 50μg/ml (Figure 2). The ethyl acetate extract, hexane extract and methanol extract both had an MIC of 100μg/ml. The DCM, DCMmethanol and acetone extracts had inhibitory activity but, however, the MICs were above the highest concentration of 100μg/ml used in the study. To compare the potency of the extracts, the percentage growth inhibition at the maximum concentration of 100μg/ml was determined and results are shown in Table 1.
Extract
Relative inhibition of Candida albicans (%)
Relative inhibition of Candida krusei (%)
DCM: Methanol
2 ± 1
58 ± 2
Hexane
29 ± 2
112 ± 6*
DCM
48 ± 1
63 ± 5
Ethyl acetate
61 ± 6
94 ± 1
Acetone
75 ± 2
45 ± 2
Methanol
90 ± 5
91 ± 6
Ethanol
85 ± 4
83 ± 2
Water
29 ± 4
6.2 ± 3
*The value of more than 100% indicates that there was growth stimulation instead of inhibition.
Table 1: The percentage decrease of cell density after exposure to 100μg/ml of plant extract.
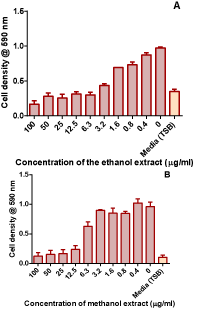
Figure 1: Antifungal activity of the ethanol and methanol extracts from
Combretum zeyheri in C. albicans. Values are the mean ± SD for 4 replicates.
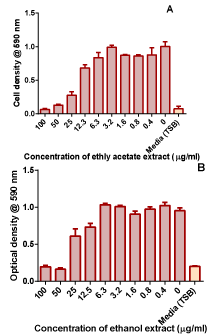
Figure 2: Antifungal activity of the ethanol and methanol extracts from
Combretum zeyheri in C. krusei. Values are the mean ± SD for 4 replicates.
Screening for an effective C. zeyheri concentration that inhibits Candida biofilm formation
The extracts were screened for their ability to inhibit biofilm formation in C. krusei and C. albicans. In C. albicans (Figure 3), methanol extract showed inhibitory activity, but the hexane, DCM and DCM-methanol extracts showed greater potency in inhibition of biofilm formation. In C. krusei (Figure 4), the water extract, methanol extract, ethyl acetate extract and hexane extract showed inhibitory activity. However, the DCM and DCM-methanol extract showed greater potency in inhibiting biofilm formation C. krusei showed greater anti-biofilm sensitivity to more leaf extracts than C. albicans. The MIC for biofilm formation of the DCM extract was determined and was found to be 500μg/ml in C. albicans and in C. krusei 62.lμg/ ml (Figure 5).
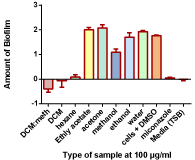
Figure 3: The effects of different leaf solvent extracts of C. zeyheri on biofilm
formation by C. albicans. Biofilm formation was determined in the 12-well
NucleonTM tissue culture plates; the intensity of the blue colour indicates
the amount of biofilm that was present in each well. The biofilm mass that
formed during the incubation period was stained using crystal violet. The
absorbance values of crystal violet are directly proportional to the amount of
biofilm that had formed. The dye was extracted using ethanol and quantified
spectrophotometrically at 590nm.
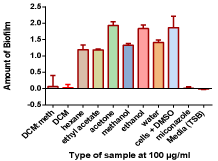
Figure 4: The effects of different leaf solvent extracts of C. zeyheri on biofilm
formation by C. krusei. Biofilm formation was determined in the 12-well
NucleonTM tissue culture plates; the intensity of the blue colour indicates the
amount of biofilm that was present in each well. The dye was extracted using
ethanol and quantified spectrophotometrically at 590nm.
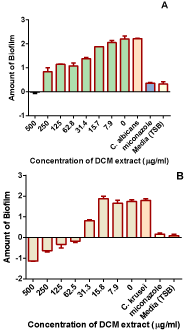
Figure 5: Determination of the MIC value for the DCM extract which inhibited
biofilm formation in C. albicans (A) and C. krusei (B).
The effects of combining potent anti-biofilm extract with a standard antifungal drug
After combining miconazole and the DCM extract, it was found that in both C. krusei and C. albicans, the combination did not improve the efficacy of the miconazole (Figure 6). Miconazole combined with the potent extract resulted in the highest biofilm formation inhibition.
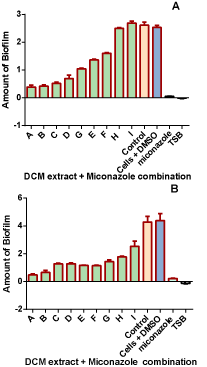
Figure 6: The effect of combining miconazole and DCM extract on biofilm
formation in C. albicans (A) and C. krusei (B).The control were cells incubated
in media.
Discussion
Biofilm formation is one of the methods used by pathogenic fungi to invade host immune response. C. albicans is now the 4th leading cause of nosocomial infections [29]. Biofilms prevent the penetration of antifungal drugs through the matrix. These biofilms results in therapy failure and recurrent fungal opportunistic infections especially in immune compromised patients [30]. The solution to this problem is to find sources of compounds which can bypass or disrupt biofilms or inhibit biofilm formation. Promising leads have been in plant extracts such as from the Combretaceae ssp [31]. This study was aimed at identifying C. zeyheri extracts which inhibit biofilm formation in C. albicans and C. krusei.
C. zeyheri plant extracts have already been shown to possess antifungal activity from other previous studies done with the plant [24,32,33]. C. krusei was more sensitive to most extracts compared to C. albicans. The minimum inhibitory concentration of miconazole in C. albicans observed was 6.3μg/ml and that of C. krusei was 3.2μg/ml. Generally C. krusei is more resistant and presents with higher MICs [34]. In this study a clinical strain of C. albicans and a laboratory strain of C. krusei were used. Clinical strains of microorganisms are usually more resistant compared to laboratory strains [35].
The extracts with the most potent activity were ethyl acetate extract, acetone extract, methanol extract and ethanol extract. These extracts seem to contain the most amounts of the active compounds with antifungal activity. In a study by Mangoyi et al., [24] the ethanol extract of C. zeyheri was shown to have antifungal activity. In the same study flavonoids from the same extract had antifungal activity. This may indicate possible presence of these active compounds in the extracts in varying amounts and possibly in higher amounts in the four most active compounds. In extracts which showed no activity it may indicate that the active compounds were absent or in minute amounts which were not able to produce an observable effect. In the study by Runyoro et al, [33], the ethyl acetate extract was active due to an isolated compound, terminolic acid. In our study, the ethyl acetate extract was biologically active in terms of inhibition of growth and biofilm formation and this could be due to the presence of terminolic acid as found in the earlier study.
The possible mechanism involved in the antifungal activity of these extracts may involve the biosynthetic pathway of ergosterol synthesis. C. zeyheri leaf ethanol extract has been reported to affect ergosterol production, although to a lesser extent compared to miconazole [23]. It is also possible that the extracts may affect the antioxidant capability of the fungi as it was also shown that the 5-hydroxy-7,4’-dimethoxyflavone from the leaf extract reduced the activities of fungal superoxide dismutase, catalase, glutathiones- transferase, glutathione peroxidase, glutathione reductase and glucose-6 phosphate dehydrogenase. Antioxidant enzymes prevent oxidative stress on the fungal cells [36].
The water extracts in particular did not show any activity in this study in both organisms. This loss of activity can be explained by synergy of the compounds in all the different extracts. Synergy refers to a combined effect of compounds to give a greater effect than either of the compounds separately [37]. Separation into different fractions could have led to loss of activity. A simple example is of Cinchona alkaloids. Almost 30 alkaloids are found in Cinchona bark, several of which are active against.
Plasmodium falciparum in vitro. The combination of quinine with quinidine and cinchonine is 2-10 times more effective in vitro against quinine-resistant strains than either of them alone [38]. This is a demonstration that at times compounds from plant are less effective when used singly. In the study by Mangoyi et al, [24], three of the isolated compounds showed less activity compared to the crude extract. Loss of activity of some compounds is, therefore, common with further separation of the crude extracts. Apart from synergy of compounds as a possible explanation to loss of activity the following factors may also have a role to play: Plant material processing, the poor quality of ethno- pharmacological studies, and degradation of active constituents during fractionation preclinical laboratory protocols which are often very different from local practices [37].
There are no previous studies on inhibition of biofilm formation in C. krusei and C. albicans using C. zeyheri extracts. The extracts that had potent antifungal activity did not necessarily inhibit biofilm formation as would be generally expected. The extracts that had less antifungal activity actually were more potent at inhibiting biofilm formation. The water extract was an exception as it showed both reduced activity in biofilm formation and antifungal activity. This is in agreement with water being used as an extractant in traditional medical practices as water based concoctions are used and are effective treatments for various ailments [39]. This result may indicate that components of the water extract work by different mechanism of action in terms of inhibition of biofilm formation and inhibition of fungal growth. Resistance mechanism involved in biofilms cannot be explained by conventional mechanism but the conventional mechanism cannot be completely disregarded. This suggests that biofilms have different intrinsic mechanism. Suggested resistance mechanisms may involve neutralizing enzymes, limited diffusion, slow growth, persistent cells and functional heterogeneity and there is less involvement of common targets such as ergosterol or glucans [40]. This may explain why some extracts in this study did not have an inhibitory effect on biofilm formation but had antifungal activity on planktonic fungal cells.
In a study by Zhou et al., [41], it was shown that ursolic acid and oleanic acid at quarter MIC inhibited biofilm formation in bacteria. Ursolic acid and oleanic acid are compounds that were isolated from C. zeyheri in the study by [33]. These compounds may also be the ones responsible for inhibition of biofilm formation in C. krusei and C. albicans in this study. In another study, with asiatic acid a compound present in C. zeyheri, it was shown to increase the sensitivity of Pseudomonas aeruginosa biofilms to tobramycin [42]. The exact mechanism of asiatic acid is not yet known, it may be related to increase in diffusion channels which could have increased penetrability of tobramycin. In another study by Ren et al, [43] ursolic acid inhibited biofilm formation in P. aeruginosa, Vibrio harveyi and E. coli. All these compounds are present in C. zeyheri extracts and this may similarly be involved in the inhibition of biofilm formation in C. krusei and C. albicans.
The combination of miconazole and DCM extract resulted in decreased biofilm formation inhibition. Miconazole and the extract alone proved effective in inhibition of biofilm formation. This was not the case as combined drug-extract had a reduced effect. The reduced activity of the combination may be explained by drug-extract incompatibility. There two types of incompatibilities: Physical and chemical. Physical reactions refer to either phase separation due to a change in ionization and solubility [44]. A chemical incompatibility refers to a chemical degradation as a result of oxidation, reduction, hydrolysis or decomposition. The physical or chemical changes of a drug may manifest as color change, precipitation or turbidity and as a result the amount of active agent diminishes [45]. It is, therefore, a possibility that miconazole and DCM extract had an incompatibility which resulted in loss of activity of miconazole and the extract.
The checkerboard data can be analyzed using mathematical models to identify or conclude no interactions, synergistic and antagonistic interactions [28]. Antifungal combinations are assessed on the basis of fractional inhibitory concentration (FIC) index. It represents the sum of the FICs for each drug. It is based on the theory that a drug similar to itself will not interact with itself and the effect is additive to give a total of 1. FIC values lower than 1, indicate synergy and those higher indicate antagonism [46] reactions could have been verified by use of mathematical models. A combination of miconazole and an isolated compound from the ethanolic extract of C. zeyheri showed great potency as an antifungal agent [24]. Reduced activity of the DCM extract and miconazole in inhibition of biofilm formation may support that mechanism involved in biofilm formation inhibition that they are different for those involved in simple inhibition of planktonic fungal cell growth. This was demonstrated by some extracts which had less growth inhibitory activity but showed more activity in biofilm formation.
Conclusion
C. zeyheri extracts inhibit biofilm formation in both C. albicans and C. krusei. The ethanol, methanol, ethyl acetate and acetone had the most antifungal activity. The antifungal activities of the extracts were less than that of miconazole. The extracts with antifungal activity were not as potent in the inhibition of biofilm formation. Extracts which had shown less antifungal activity showed more activity in inhibition of biofilm formation. It can also be concluded that antifungal activity may not necessarily indicate ability to inhibit biofilm formation. Combining miconazole and the potent leaf extract did not augment the anti-biofilm activities.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Acknowledgement
Support from the International Science Programmes (ISP) through the International Program in the Chemical Sciences (ISP IPICS: ZIM01, Uppsala University, Uppsala, Sweden) and the International Foundation in Sciences (IFS F/3413-03F, Stockholm, Sweden) us acknowledged. F/3413-03F supported research under the title: “Screening natural plant products from selected plants from Zimbabwe as a source of anti-infective compounds for phytomedicines development”. ISP IPICS: ZIM01 supported the research under the title “Biomolecular Interactions Analyses”. The authors acknowledge the assistance of Mr. Christopher Chapano, a taxonomist with the National Herbarium and Botanical Gardens, Harare, Zimbabwe in the authentication of the plant sample names.
References
- Davies J and Davies D. Origins and Evolution of Antibiotic Resistance. Microbiol Mol Biol Rev. 2010; 74: 417–433.
- Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, et al and for the World Healthcare-Associated Infections Forum participants. Antimicrobial Resistance and Infection Control. 2013.
- Dar SA, Yousuf AR, Ganai FA, Sharma P and Kumar N. Bioassay guided isolation and identification of anti-inflammatory and anti-microbial compounds from Urtica dioica L. (Urticaceae) leaves. African J. Biotechnol. 2012; 11: 12910-12920.
- Alabi OA, Haruna MT, Anokwuru CP, Jegede T, Abia H, Victor U, et al. Comparative studies on antimicrobial properties of extracts of fresh and dried leaves of Carica papaya (L) on clinical bacterial and fungal isolates. Adv Appl Sci Res. 2012; 3: 3107-3114.
- Mokgoatsane ST. The isolation and characterization of an antibacterial compound from Terminalia sambesiaca (Combretaceae). Magister Scientiae (MSc) thesis. North West University. School of Pharmacy. 2011.
- Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, et al. The Global Antifungal Surveillance Group. 2008. Candida krusei, a Multidrug- Resistant Opportunistic Fungal Pathogen: Geographic and Temporal Trends from the Artemis Disk Antifungal Surveillance Program. 2001 to 2005. J. Clin Microbiol. 2008; 46: 515-521.
- Shao LC, Sheng CQ and Zhang WN. Recent advances in the study of antifungal lead compounds with new chemical scaffolds. Yao Xue Xue Bao. 2007; 42: 1129-1136.
- Sorgo AG, Heiman CJ, Brul S, de Koster CG, Klis FM. Beyond the wall: Candida albicans secret to survive. FEMS Microbiol. Lett. 2013; 338: 10-17.
- Hosta B. Contamination, disinfection, and cross colonization: Are hospitals surfaces reservoirs for nosocomial infection? Clin Inf Diseases. 2004; 39: 1182-1189.
- Berman J and Sudbery PE. Candida albicans: A molecular revolution built on lessons from budding yeast. National Review Genetics. 2002; 3: 918-930
- Naglik JR., Challacombe SJ and Hube B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003; 67: 400-428
- Donlan RM. Biofilms: Microbial Life on Surfaces. Emerg Infect Dis. 2002; 8: 881-890.
- Prakash B, Veeregowda BM and Krishnappa G. Biofilms: A survival strategy of bacteria. Current Science. 2003; 85: 1299-1307.
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW and Azeredo J. Adherence and biofilm formation of non-Candida albicans species. Trends in Microbiology. 2011; 19: 241-247.
- Davey ME and O’toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiology: Molec Biol Rev. 2000; 64: 847-867.
- Cragg GM and Newman DJ. Natural products: A continuing source of novel drug leads. Biochim Biophys Acta. 2013; 1830: 3670-3695.
- Fyhrquist P, Mwasumbi L, Vuorela P, Vuorela H, Hiltunen R, Murphy C, et al. Preliminary antiproliferative effects of some species of Terminalia, Combretum and Pteleopsis collected in Tanzania on some human cancer cell lines. Fitoterapia. 2006; 77: 358-366.
- Breytenbach JC and Malan SF. Pharmacochemical properties of Combretum zeyheri. South African Journal Sci. 1989; 85: 372-374.
- Cheng HY, Lin TC, Yu KH, Yang CM and Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula. Biology Pharmaceutical Bulleting. 2003; 26: 1331-1335.
- https://innovareacademics.in/journal/ijpps/Vol5Suppl4/7875.pdf
- Runyoro D, Santosh S, and Mahendrah P. Anticandidiasis agents from a Tanzanian plant, Combretum zeyheri. Med Chem Res. 2013; 22: 1258 -1262.
- Mangoyi R, Mafukidze W, Marobela K, and Mukanganyama S. Antifungal activities and preliminary phytochemical investigation of Combretum species from Zimbabwe. J. Microbial Biochem Tech. 2012; 4: 37-44.
- Mutasa T, Mangoyi R and Mukanganyama S. The effects of Combretum zeyheri leaf extract on ergosterol synthesis in Candida albicans. J. Herbs. Spices and Medicinal Plants. 2015; 21: 211-217.
- Mangoyi R, Midiwo J and Mukanganyama S. Isolation and characterization of an antifungal compound 5-hydroxy-7, 4’-dimethoxyflavone from Combretum zeyheri. BMC Complementary and Alternative Med. 2015; 5: 405.
- Washington JA, Warren E and Karlson AG. Stability of barium sulfate turbidity standards. Appl Microbiol. 1972; 24: 1013.
- Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes- Giannini MJS. Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus spp. Rev. Ciênc. Farm. Básica Apl. 2007; 28: 25-34.
- Borucki MK, Peppin JD, White D, Loge F and Call DR. Variation in Biofilm Formation among Strains of Listeria monocytogenes. Appl Environ Microbiol. 2003; 69: 7336-7342.
- Eliopoulos G and Moellering Jr RC. Antimicrobial combinations. In V. Lorian (ed). Antibiotics in laboratory medicine. The Williams & Wilkins Co. Baltimore MD. 1996: 330-396.
- Pfaller MA and Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Res Microbiol. 2010; 36: 1-53.
- Usharani A, Bharati M and Sandhya C. Isolation and characterization of Candida species from oropharyngeal secretions of HIV positive individuals. NASZA Dermatology Online. 2011; 2: 119-124.
- Bag A, Bhattacharyya SB and Chattopadhyay RR. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac J Trop Biomed. 2013; 3: 244-252.
- Mangoyi R and Mukanganyama S. In vitro antifungal activities of some selected plants from Zimbabwe against Candida albicans and Candida krusei. AJPSB. 2011; 3: 8-14.
- Runyoro DKB, Srivastava SK, Darokar MP, Olipa ND, Joseph CC and Matee MIN. Anticandidiasis agents from a Tanzanian plant, Combretum zeyheri. Med Chem Res. 2013; 22: 1258–1262.
- Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, eCuenca-Estrella M, et al. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLOS ONE. 2013; 8: e60047.
- Adamus-Bialek W, Zajac E, Parniewski P and Kaca W. Comparison of antibiotic resistance patterns in collections of Escherichia coli and Proteus mirabilis uropathogenic strains. Mol Biol Rep. 2013; 40: 3429-3435.
- Gonz´alez-Parraga P, Hernandez JA and Arguelles JC. Role of antioxidant enzymatic defences against oxidative stress (H2O2) and the acquisition of oxidative tolerance in Candida albicans. Yeast. 2003; 20: 1161-1169.
- Rasoanaivo P, Wright CW, Willcox ML and Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011; 10: S4.
- Karle JM and Bhattacharjee AK. Stereoelectronic features of the cinchona alkaloids determine their differential antimalarial activity. Bioorganic Med Chem. 1999; 7: 1769-1774.
- Martini ND, Katerere DRP and Eloff JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnomed. 2004; 93: 207-212.
- Pagán-Mercado G, Rivera-Ruiz ME, Segarra-Román F and Rodríguez- Medina JR. Antifungal Research Strategies Aiming for New Targets. P R Health Sci J. 2009; 28: 220-226.
- Zhou L, Ding Y, Chen W, Zhang P, Chen Y, and X Lv. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Diseases. 2013; 19: 494-500.
- Garo E, Eldridge GR, Goering MG, DeLancey Pulcini E, Hamilton MA, Costerton JW, et al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob Agents Chemother. 2007; 51: 1813-1817.
- Ren D, Zuo R, González Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol. 2005; 7: 4022- 4034.
- Newton D. Drug incompatibility chemistry. Am J Health Syst Pharm. 2009; 66: 348-357.
- Höpner JH, Schulte A, Thiessen J, Knuf M and Huth RG. Preparation of a compatibility chart for intravenous drug therapy in neonatal and pediatric intensive care units. Klin Padiatr. 2007; 219: 37-43.
- Meletiadis J, Pournaras S, Roilides E and Walsh TJ. Defining fractional inhibitory concentration index cutoffs for additive interactions based on selfdrug additive combinations, Monte Carlo Simulation Analysis, and in vitroin vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrobial Agents Chemotherapy. 2010; 54: 602-609.