
Research Article
J Bacteriol Mycol. 2018; 5(3): 1069.
Lemongrass Oil Components Synergistically Activates Fluconazole against Biofilm Forms of Candida albicans
Pandurang MS, Devrao HS, Ganpatrao BR, Mohan KS*
¹School of Life Sciences (DST-FIST & UGC-SAP Sponsored), SRTM University, Nanded, India
*Corresponding author: Karuppayil Sankunny Mohan, School of Life Sciences, SRTM University, Nanded, India
Received: May 02, 2018; Accepted: June 04, 2018; Published: June 11, 2018
Abstract
Background of Research: Device related infections caused Biofilm forms of Candida albicans are life threatening because of their increased tolerance of antifungal drugs. Combinatorial therapy involving phytochemicals and antifungal drugs would be an effective approach against drug resistant Candida biofilms. Some of the components of lemongrass oil were tested in combination with fluconazole against planktonic and biofilm forms of C. albicans ATCC 90028 and one clinical isolate.
Methods: Activities of various combinations were analyzed by chequerboard format. The interaction between lemongrass oil components with fluconazole were determined by calculating fractional inhibitory concentration indices (FICI) and results obtained by XTT metabolic assay were confirmed by scanning electron microscopy. Result: Components of lemongrass oil, like Nerol, Citral, Linalool, β-ionone, Terpinolene, 1,8 Cineol, Geraniol & Geranyl acetate in combination with fluconazole showed synergistic interaction against developing biofilm of C. albicans. Whereas Geraniol, Nerol and β-ionone with fluconazole combination showed synergistic interaction against mature biofilms of C. albicans.
Conclusion: This study for the first time indicates that lemongrass oil components increases efficacy of fluconazole against biofilms of C. albicans.
Keywords: Candida albicans; Synergistic; Biofilm; Combination; Drug resistance; Lemongrass oil
Abbreviations
MIC: Minimum Inhibitory Concentration; SEM: Scanning Electron Microscopy; YPD: Yeast-Peptone-Dextrose, PBS: Phosphate Buffer Saline; MOPS: 3-[N-morpholine] Propane Sulphonic Acid; RPMI: Roswell Park Memordium Institute; DMSO: Dimethyl Sulphoxide; XTT: 2,3-bis (2-methoxy-4-nitro-sulfophenyl)- 2H-tetrazolium-5-carboxanilide; CLSI: Clinical and Laboratory Standards Institute; FLZ: Fluconazole; FICI: Fractional Inhibitory Concentration Indices.
Introduction
Candida albicans, an important pathogen of the humans can form drug resistant biofilms on biotic as well as a biotic surfaces [1,2]. It is the most common hospital-acquired infectious agent. C. albicans may cause superficial and serious systemic mycosis. Biofilm formation on prosthesis devices like, heart valves, stents or indwelling medical devices is a serious clinical challenge. Many of the prescribed antifungal drugs are ineffective against biofilms [3,4]. Antifungal agents like azole group of drugs target ergosterol synthesis in the cell membrane of Candida albicans, while some studies showed that the level of ergosterol is significantly decreased in intermediate and mature phase of biofilm growth compared to those in developmental phase [5,6]. Antifungal agents available in the market are ineffective because of the development of multifactorial drug resistance mechanisms. The mature biofilm may act as a source of re-current infections, so there is a need to identify better drug targets for eradication of mature biofilm [7].
There is considerable interest in using essential oils and their components as alternatives to synthetic antifungal drugs. Lemongrass (Cymbopogoncitratus) is used since ancient times in traditional medicine in many countries. Lemongrass oil has different properties like antibacterial, antifungal activities as well as analgesic and antiinflammatory activities [8]. The antifungal activities of essential oil of Lemongrass (Cymbopogoncitratus) are reported against C. albicans [9]. Lemongrass oil contains 12 to 15 components of Terpenoids and Terpene in nature. The chemical composition of the Lemongrass oil is Geraniol (0.72%), Linalool (3.82%), Limonene (4.12%), Terpinolene (1.52%), 1-8cineole (0.12%), Myrcene (18.5%), Geranyl acetate (0.23%), Nerol (23.92%), Geranial (43.2%). β-Ionone (0.03%), β-citronellol (2.05%), a pinene (0.06%), β-caryophyllene (2.05%) and Citral is an combination of neral plus geranialreported by Farhang, et al (2013), Wagara, et al (2011) and Sulaiman, et al (2013) [10,11,8].
Many terpenoid molecules of plant origin have promising activity against growth and the virulence factors of C. albicans [12,13]. A main characteristic of C. albicans biofilm is resistance to various antifungal such as the widely used antifungal drug fluconazole. High dosages of antifungal drugs are not recommended due to increased toxicity as side effect. To limit the use of high concentration of antifungal drugs, drug combination strategy is explored against biofilm associated C. albicans infections [14,15]. To study the effect of these components with fluconazole a combination study is done. Phytochemicals are being proposed as candidates for Synergy research to generate new pharmaceuticals [16]. Combination therapy is very useful and effective therapy for increasing the efficacy of drug towards the resistant strain of pathogen. Rate and degree of microbial killing could be increased by using combinational approach [17].
Materials and Methods
A culture of Candida albicans (ATCC 90028), was obtained from the Institute of Microbial Technology, Chandigarh, India. One clinical isolates was obtained from Government Hospital, Parbhani, Maharashtra, India. Both the cultures were maintained on yeast extract peptone dextrose (YPD) agar slants at 40C (HiMedia laboratories Pvt. Ltd. Mumbai India). A single colony from YPD agar plate was inoculated in 50ml of YPD broth and incubated at 30°C temperature, on a shaking (120rpm) incubator for 24h. Cells from the activated culture were collected by centrifugation at 2000g speed for 5min, washed thrice and resuspended in PBS (pH 7.4) for further experimentation. RPMI-1640 medium (w/L-glutamine, w/o sodium bicarbonate; pH7, buffered with 165mM MOPS (3-[N-morpholine] propane sulphonic acid) was filter sterilized and used as the assay medium. Various concentrations of components of lemongrass oil were prepared in RPMI by double dilution method. Concentration of the solvent i.e. Dimethyl Sulphoxide (DMSO), never exceeded 1%. Fluconazole was used as a standard antifungal drug.
All media components and chemicals were purchased from HiMedia laboratories Pvt. Ltd. Mumbai. XTT [i. e. 2,3-bis (2-methoxy-4-nitro-sulfophenyl)-2H-tetrazolium-5-carboxanilide] and menadione were procured from Sigma-Aldrich Chem. Ltd. Components of lemongrass oil like Citral, Nerol, Linalool, 1,8 cineole, Geraniol, Β-citronellol, Myrcene, Geranyl acetate, limonene (all analytical grade) were obtained from Sigma-Aldrich Chemicals Ltd, Mumbai, India. β-Ionone as well as other media components was purchased from HiMedia laboratories Pvt. Ltd. Mumbai, India.
Minimum inhibitory concentration/minimum fungicidal concentration
The effect of drug combinations on the growth of C. albicans was studied using the standard broth micro dilution methodology based on the Clinical Laboratory Standards Institute guidelines [18]. Various concentrations of test molecules ranging from 0.031 to 4 mg/ ml were prepared in RPMI-1640 medium in 96 well plates (Costar, Corning Inc., USA). Wells without test compounds was served as controls, while Fluconazole (1–256µg/ml) was used as a standard antifungal agent. One hundred micro liter of inoculum was added to 100µl of RPMI-1640 medium in each well to obtain 1X103cells/ ml. The plates were incubated at 35°C for 48h. To analyze growth, the absorbance was read at 620nm using a microplate reader (Multiskan EX, Thermo Electron Corp., USA). The lowest concentration of the test compound which will cause a 50% reduction in the absorbance compared to the control was considered as the minimum inhibitory concentration (MIC) for growth of Candida albicans.
Molecule for which the MIC was achieved (in the range 0.031 to 4 mg/ml) was selected for minimum fungicidal concentration (MFC) testing. To determine the MFC with respect to Candida, cells from the MIC and wells containing concentrations above the MIC was used. Aliquots of 10µl from these wells were spread on YPD agar. These plates was incubated for 48h at 30°C and observed for the presence of colonies. No appearance of colonies on the agar plates was noted as a fungicidal effect [18]. The lowest concentration of the test molecule in the microplate well from which an aliquot showing no growth was considered as the MFC.
Biofilm Formation and drug susceptibility
For biofilm formation, 100μl of the cell suspension (1×107cells/ ml in PBS) were added to each well of 96 well microtitre plates. Plates were incubated at 370c on an orbital shaker for 90 minutes of adhesion phase. Wells were washed with sterile PBS to remove non-adhered cells and 200μl of RPMI for-1640 medium was added to adhered cells. To carry out susceptibility of biofilm development, medium containing various concentration of the drugs were added at the zero hour of biofilm formation i.e. immediately after adhesion phase and the plates were incubated at 370c for 48hr, at 100rpm in an orbital shaker. While, to analyse the effects on mature biofilms, medium with a range of drug concentration were added to the 24hr. mature biofilms the plates were further incubated for 48 hour at 370C. Density of the cells survived in biofilm forms were analysed through metabolic activity in XTT formazan reduction assay [19].
Biofilm Quantitation by XTT assay
Biofilm formation was quantitated using XTT [i.e. 2,3-bis (2- methoxy-4nitro-sulfophenyl)-2H-tetrazolium-5-carboxanilide] (sigma-Aldrich, India) reduction assay. Briefly, XTT solution was prepared by mixing 1mg/ml XTT salt in PBS and stored at -20c. Prior to use, menadione solution was prepared in acetone (sigma- Aldrich, India)was added to XTT to a final concentration of 4µM. The wells containing biofilm was washed with PBS to remove non adhered cells and incubated for 5 hour in 100µl of XTT–menadione solution in dark, at 370c at 100rpm. The colour formation by water soluble formazan product was measured at 450nm using a microplate reader. Wells without biofilms were served as a blank [19].
Checkerboard format for determination of FICI
Combinatorial efficacy of the components of lemongrass oil and Fluconazole were analyzed in terms of fractional inhibitory concentration indices (FICI) obtained in checkerboard assay. Dilutions of Fluconazole and components of lemongrass oil as well as their combinations were prepared in a checkerboard format as per standard methodology [17]. A two dimensional array of serial concentrations of test compounds was used for preparation of dilutions of the drugs. For planktonic growth, the micro plates were incubated at 35°C, while for biofilm growth the plates were incubated at 37°C for 48 hours. MICs for planktonic and biofilm growth were determined as mentioned in previous sections.
FICI values were calculated using formula:
£FIC = FICA+ FICB,
Where FICA= (MIC of drug A in combination/MIC of drug A alone),
FICB= (MIC of drug B in combination/MIC of drug B alone).
When the value of £FIC = 0.5 it is the synergism; between 0.5 and 1.0 it is additive; and when £FIC > 4 it is known as the antagonism. A FIC result of > 1.0 but =4 is considered as indifference [20].
Microscopic analysis of Candida albicans and Biofilm
Cells were observed under an inverted light microscope (Metzer, India). Photographs were taken by a Labomed microphotography system (LabomedKorntal, Germany).For scanning electron microscopy (SEM), samples were fixed in 2.5% glutaraldehyde in 0.1mol 1-1 phosphate buffer (ph 7.2) for 24 hour at 4°C samples was post fixed in 2% aqueous solution of osmium tetra oxide for 4 hour, then dehydrated in a series of graded alcohols and finally dried stubs, and gold coating were performed using an automated gold coater for 3min. biofilms were observed under an inverted light microscope[19].
Statistical analysis
Values mentioned are the mean with standard deviations, obtained from three different observations. Values in the control and treatment groups for various molecules were compared using Student’s t -test. A value of P< 0.05 was considered statistically significant [18].
Results
Lemongrass oil Components sensitizes Candida albicans to fluconazole
Components of lemongrass oil like, citral, Nerol, geraniol, linalool, 1,8cinole, terpinolene, ionone, and limonene and geranyl acetate showed MICs against planktonic growth of both the strains of C .albicans. At concentrations above 2mg/ml geraniol, citronellol and linalool exhibited inhibitory activity against C. albicans. 1,8-cineole, Nerol and terpinolene required high concentration for inhibition i.e. above 4mg/ml. Citral, ionone and geranyl acetate were highly effective at concentrations above 0.5mg/ml. A significant decrease in MICs of fluconazole was observed when cells were exposed to combination of Fluconazole with Lemongrass oil components against C albicans ATCC 90028 and one clinical strain (isolate). For example, the MIC of fluconazole in combination with limonene was 0.125mg/ml concentration and limonene was found to be 0.5mg/ml concentration. The FICI value for Limonene-fluconazole combination was calculated to be 0.4, which indicated that the combination is synergistic. Similarly as per observation table (Table 1) the calculated FICI value for the other oil components like Citral, Geraniol, Nerol, Linalool, Citronellol and 1,8 cineole with fluconazole in combination is below 0.5. It is indicated that the combination is Synergistic against C. albicans ATCC 90028.Similarly Citral, Nerol, Citronellol, β-ionone and Geraniol combination with fluconazole were showed synergistic interaction against Clinical isolate of C. albicans. Whereas the treatment of β-ionone & terpinolene with fluconazole together was not synergistic. FICI value of β-ionone fluconazole combination is 0.628. Terpinolene fluconazole combination showed FICI value of 0.6, indicating additive effect against C. albicans. 1,8 cineol, Terpinolene, Linalool and limonene in combination with fluconazole were additive effect. Geranyl acetate combination with fluconazole showed Indifference effect, FICI value for this combination is 1.064 against both strain of C. albicans (Table 1 and 2).
Fluconazole Combination with Limonene
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
limonene
FLZ
limonene
Planktonic
0.1
2
0.125
0.5
0.4
Synergistic
Biofilm development
0.512
>4
256
8
1
Indifference
Mature Biofilm
>1.024
>4
256
8
1.1
Indifference
Fluconazole combination with Nerol
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Nerol
FLZ
Nerol
Planktonic
0.001
4
0.00025
1
0.5
Synergistic
Biofilm development
0.512
0.5
0.004
0.031
0.1
Synergistic
Mature Biofilm
>1.024
2
0.032
0.5
0.26
Synergistic
Fluconazole combination with Citral
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Citral
FLZ
Citral
Planktonic
0.001
0.5
1.5E-05
0.5
0.5
Synergistic
Biofilm development
0.512
0.5
0.008
0.139
0.1
Synergistic
Mature Biofilm
>1.024
2
0.256
2
1.125
Indifference
Fluconazole combination with Linalool
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Linalool
FLZ
Linalool
Planktonic
0.001
1
3.1E-05
0.125
0.156
Synergistic
Biofilm development
0.512
0.5
0.016
0.5
0.3
Synergistic
Mature Biofilm
>1.024
1
0.004
2
2
Indifference
Fluconazole combination with Geraniol
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Geraniol
FLZ
Geraniol
Planktonic
0.001
1
0.25
0.125
0.5
Synergistic
Biofilm development
0.512
1
0.125
0.125
0.187
Synergistic
Mature Biofilm
>1.024
2
0.0016
0.25
0.133
Synergistic
Fluconazole combination with citronellol
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Citronellol
FLZ
citronellol
Planktonic
0.001
1
0.00013
0.062
0.187
Synergistic
Biofilm development
0.512
1
0.256
0.25
0.75
Additive
Mature Biofilm
>1.024
2
0.128
2
1.06
Indifference
Fluconazole combination with Β-Ionone
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Β Ionone
FLZ
Β Ionone
Planktonic
0.001
0.25
0.5
0.032
0.628
Additive
Biofilm development
0.512
1
0.064
0.125
0.132
Synergistic
Mature Biofilm
>1.024
2
0.032
0.5
0.266
Synergistic
Fluconazole combination with 1,8 Cineol (Eucalyptol)
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
1, 8 Cineol
FLZ
1, 8 Cineol
Planktonic
0.001
>4
1.5E-05
1
0.3
Synergistic
Biofilm development
0.512
>4
0.032
2
0.75
Additive
Mature Biofilm
>1.024
>4
0.0256
2
0.625
Additive
Fluconazole combination with Geranyl acetate(GA)
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
GA
FLZ
GA
Planktonic
0.001
0.5
0.001
0.032
1.064
Indifference
Biofilm development
0.512
4
0.016
0.5
0.156
Synergistic
Mature Biofilm
>1.024
4
0.0256
2
1.1
Indifference
Fluconazole with Terpinolene
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Terpinolene
FLZ
Terpinolene
Planktonic
0.001
4
0.0005
0.25
0.6
Additive
Biofilm development
0.512
2
0.128
0.125
0.312
Synergistic
Mature Biofilm
>1.024
4
0.128
1
0.75
Additive
Table 1: MIC of Ten Components of Lemongrass oil with Fluconazole alone as well as in combination against planktonic and biofilm growth forms of Candida albicans ATCC90028.
Fluconazole Combination with Limonene
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
limonene
FLZ
limonene
Planktonic
0.0005
2
0.25
0.25
0.63
Additive
Biofilm development
0.512
>4
4
2
0.5
Synergistic
Mature Biofilm
>1.024
>4
64
4
1.03
Indifference
Fluconazole combination with Nerol
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Nerol
FLZ
Nerol
Planktonic
0.0005
2
0.00125
0.125
0.38
Synergistic
Biofilm development
0.512
2
0.004
1
0.5
Synergistic
Mature Biofilm
>1.024
4
0.256
1
0.37
Synergistic
Fluconazole combination with Citral
Candida albicans
ALONE in MIC mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Citral
FLZ
Citral
Planktonic
0.0005
1
0.00013
0.5
0.5
Synergistic
Biofilm development
0.512
1
0.004
0.5
0.507
Synergistic
Mature Biofilm
>1.024
4
0.0032
1
0.26
Synergistic
Fluconazole combination with Linalool
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
linalool
FLZ
Linalool
Planktonic
0.0005
2
2.5E-05
0.25
0.6
Additive
Biofilm development
0.512
2
0.032
0.5
0.312
Synergistic
Mature Biofilm
>1.024
4
0.008
1
0.253
Synergistic
Fluconazole combination with Geraniol
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Geraniol
FLZ
Geraniol
Planktonic
0.0005
0.5
6.2E-05
0.062
0.12
Synergistic
Biofilm development
0.512
4
0.064
4
1.125
Indifference
Mature Biofilm
>1.024
4
0.064
0.5
0.156
Synergistic
Fluconazole combination with citronellol
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Citronellol
FLZ
citronellol
Planktonic
0.0005
2
0.0005
0.125
0.56
Synergistic
Biofilm development
0.512
4
0.128
2
0.75
Additive
Mature Biofilm
>1.024
4
0.512
4
1.25
Indifference
Fluconazole combination with Β-Ionone
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
Β-Ionone
FLZ
Β-Ionone
Planktonic
0.0005
0.5
0.0005
0.062
0.2
Synergistic
Biofilm development
0.512
4
0.032
4
1
Indifference
Mature Biofilm
>1.024
4
0.064
2
0.53
Synergistic
Fluconazole combination with 1,8 Cineol (Eucalyptol)
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
1, 8 Cineol
FLZ
1, 8 Cineol
Planktonic
0.0005
2
0.0005
0.25
0.75
Additive
Biofilm development
0.512
>4
0.128
0.25
0.312
Synergistic
Mature Biofilm
>1.024
>4
0.128
4
1.06
Indifference
Fluconazole combination with Geranyl acetate(GA)
Candida albicans
ALONE MIC in mg/ml
MIC in combination mg/ml
FICI
Remark
FLZ
GA
FLZ
GA
Planktonic
0.0005
2
0.0005
0.125
1.064
Indifference
Biofilm development
0.512
4
0.256
2
1
Indifference
Mature Biofilm
>1.024
4
0.032
1
0.265
Synergistic
Fluconazole with Terpinolene
Candida albicans
ALONE in MIC mg/ml
in combination
FICI
Remark
FLZ
Terpinolene
FLZ
Terpinolene
Planktonic
0.0005
2
0.00025
0.25
0.6
Additive
Biofilm development
0.512
2
0.008
0.25
0.14
Synergistic
Mature Biofilm
>1.024
4
0.256
1
0.375
Synergistic
Table 2: MIC of Ten Components of Lemongrass oil with Fluconazole alone as well as in combination against planktonic and biofilm growth forms of clinical isolate of Candida albicans.
Lemongrass oil Components prevented development of biofilms in C. albicans
Fluconazole was completely ineffective against biofilm formation. Significant biofilm growth was observed even at drug concentration 250 times higher (i.e.0.512 mg/ml) than the MIC of planktonic growth. Nerol, Β-ionone, Citral, Linalool, Geraniol, Citronellol and Terpinolene were active inhibitors of biofilm development with concentrations as low as 0.5-2 mg/ml. Limonene, 1,8 cineole & Geranyl acetate were ineffective & restricted biofilm development at concentrations above 4mg/ml.
In combination with fluconazole, limonene & citronellol showed indifference interaction against developing biofilm of C. albicans. Limonene fluconazole combination showed FICI index of 1, which predicted as indifference against C. albicans ATCC 90028. Similarly Geraniol, Geranyl acetate and β-ionone combination with fluconazole showed indifference against clinical isolate. In the present investigation, prevention 0f both strains of C. albicans, citronellol with fluconazole combination was showed FICI index 0.75 predicted additive effect against developing biofilms. Whereas other components like Nerol, Citral, Linalool, β-ionone, Terpinolene, 1,8 cineol, Geraniol & Geranyl acetate in combination with fluconazole showed synergistic interaction against developing biofilm of C. albicans ATCC 90028 and brought down the fluconazole MIC. For example, FICI of Nerol fluconazole combination was 0.1 & Nerol brought down the fluconazole MIC to 0.004mg/ml. Similarly, citral fluconazole combination brought down the Fluconazole MIC to 0.008mg/ml. Linalool fluconazole combination of FICI was 0.3 & linalool brought down the Fluconazole MIC to 0.016mg/ml. Geraniol fluconazole combination brought down the Fluconazole MIC to 0.032mg/ml. β-ionone fluconazole combination brought down the Fluconazole MIC to 0.064mg/ml. 1,8 cineol fluconazole combination of FICI was 0.562 & this combination brought down the Fluconazole MIC to 0.032mg/ml. Geranyl acetate fluconazole combination brought down the Fluconazole MIC to 0.016mg/ml. Terpinolene fluconazole combination brought down the Fluconazole MIC to 0.128mg/ml. Also, the combined effect of terpinolene, 1,8 cineol, linalool, citral, Nerol and limonene with fluconazole were showed synergistic interaction against clinical isolate. The biofilm preventive synergistic effects of these combinations against C. albicans ATCC 90028 were confirmed by SEM analysis (Figure-1 and Figure-2).
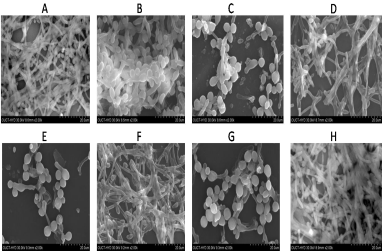
Figure 1: SEM images showing synergistic effect of lemongrass oil component with Fluconazole against biofilm formation C. albicans. This picture represents
the scanning electron micrographs under conditions. A is control biofilm; B is Fluconazole (0.512mg/ml) alone effect on biofilm; C is treatment of geraniol with
Fluconazole on biofilm (0.125mg/ml); D is alone effect of geraniol (0.125mg/ml); E is an treatment of Ionone with Fluconazole on biofilm (0.125mg/ml + 0.064mg/
ml); F is alone effect of Ionone (0.125mg/ml); G is an treatment of linalool with Fluconazole on biofilm (0.125mg/ml + 0.016mg/ml); H is alone effect of linalool
(0.125mg/ml).
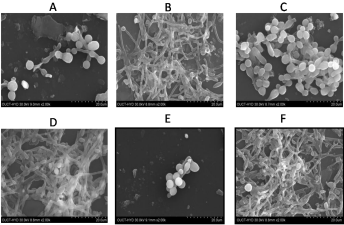
Figure 2: SEM images showing synergistic effect of lemongrass oil component with Fluconazole against biofilm formation C. albicans. This picture represents the
scanning electron micrographs under conditions. A is an treatment of Nerol with Fluconazole on biofilm (0.031mg/ml Nerol+ 0.032mg/ml FLZ; B is alone effect of
Nerol (0.031mg/ml); C is treatment of Citral with Fluconazole on biofilm (0.062mg/ml + 0.008mg/ml); D is alone effect of Citral (0.062mg/ml); E is an treatment of
Geranyl acetate with Fluconazole on biofilm (0.5mg/ml + 0.016mg/ml); F is alone effect of Geranyl acetate on biofilm formation (0.5mg/ml).
Lemongrass oil Components with Fluconazole Combinations were less effective against Mature Biofilms of C. albicans ATCC 90028 and one clinical isolate
Mature Biofilms were completely resistant to fluconazole. At very high concentration (1.024mg/ml) fluconazole is very insensitive to mature biofilms. Citral, Geraniol, Linalool and &beta Ionone moderately inhibited mature biofilm of Candida albicans. Fluconazole combined with component of lemongrass oil, like Geraniol, Nerol& Ionone showed synergistic interaction against mature biofilms. In Nerol fluconazole combination of FICI was 0.26 & it brought down the fluconazole MIC up to 0.032mg/ml. Similarly, β-ionone fluconazole combination brought down the fluconazole MIC up to 0.032mg/ml. Lastly Geraniol fluconazole combination brought down the fluconazole MIC up to 0.0016mg/ ml. Other components like limonene, citral, linalool, citronellol and geranyl acetate in combination with fluconazole showed FICI was 1 which indicates indifference interaction against mature biofilms of C. albicans ATCC 90028. Whereas 1, 8 cineole and terpinolene with fluconazole combination showed additive effect with FICI was 0.75. The FICI values =1 indicated there is no useful interaction found between limonene, citral, geranyl acetate, linalool, citronellol, 1, 8cineole and terpinolene with fluconazole against matures biofilms of C. albicans. Lemongrass oil components other than geranyl acetate, 1,8 cineol, β ionone and limonene combined with fluconazole were showed synergistic interaction against clinical isolate and these four molecules combined treatment with fluconazole were showed indifference (Table 1 and 2).
Discussion
In this study we found that components of lemongrass oil acted synergistically with fluconazole against planktonic as well as biofilm forms of C. albicans. A significant decrease in MICs of fluconazole was observed when the cells were exposed to a combination of Fluconazole with Lemongrass oil components against planktonic forms of C. albicans. The calculated FICI value for the oil components Citral, Geraniol, Nerol, Linalool, Citronellol and 1,8 cineole with fluconazole combinations are =0.5 indicating that the combination is Synergistic.
Whereas Nerol, Citral, Linalool, Β-ionone, Terpinolene, 1,8 cineol, Geraniol & Geranyl acetate in combination with fluconazole showed synergistic interaction against developing biofilms of two strains of C. albicans and brought down the fluconazole MICs from 0.256 to 0.064 mg/ml, 0.016mg/ml, 0.08mg/ml. Fluconazole combined with Geraniol, Nerol & Ionone showed synergistic interaction against mature biofilms reducing fluconazole MICs to 0.032mg/ml or 0.0016mg/ml.
It is reasonable to assume that fluconazole in combination with lemongrass oil components, appears to affects the activity of different enzymes responsible for the biosynthesis of ergosterol. During process of inhibition of ergosterol biosynthesis toxic methylated sterols are accumulated and thereby fungal cell growth arrested [21]. Lemongrass oil components pass through the fungal cell wall and disrupted the plasma membrane structure of these yeasts, rendering them more permeable to uptake of fluconazole. So, increased influx of fluconazole with lemongrass oil components causes leakage of cell content essential for growth of C. albicans and showed synergistic interaction against biofilms forms of C. albicans.
Phytocompoundes are reported to destabilize membrane and regulate membrane associated functions like permeability and cell signaling leading to cell death [18]. The anticandida activity of lemongrass oil components is considered to be due to membrane damage as well as inhibition of oxidative phosphorylation & respiratory chain function. Again terpenoids are reported to modulate the mevalonate pathway and affect cell membrane synthesis [22]. Another possibility is that terpenoid components target cell cycle progression & block the cells in specific stages of cell cycle to inhibit Candida albicans. Lemongrass oil components like citral, citronellol, geraniol and geranyl acetate are reported to block C. albicans in S phase of cell cycle [23]. Geraniol, a terpenoid present in lemongrass oil was found to interfere in membrane functions, ion homeostatic as well as cell signaling event. Limonene is known to arrest cell division, modulate cell signaling pathways and affect cell growth in cancerous cells [22]. Linalool inhibits hyphae growth or biofilm formation via inhibiting cAMP-PKA pathway [24]. Combination of these plant molecules with antifungal drugs with different mode of action may inhibit multiple targets and hence would be a good strategy against biofilms.
A combinatorial approach in antifungal therapy offers several advantages like increased potency, reduced dosages of antifungal drugs, lowered toxicities, prevention of the drug resistance and eradication of biofilms [24,25]. Terpenoids are fungicidal in nature and it increases the sensitivity of C. albicans biofilms towards fluconazole by altering the fungi static activity into fungicidal. This is the first study that reports on the synergistic effect of lemongrass oil components in combination with fluconazole and its potential use to treat infectious diseases caused due to biofilms of C. albicans.
Conclusions
The outcome of this in vitro study suggest use of lemongrass oil components with antifungal drugs as a strategy for prevention of Candida albicans biofilm & also for avoidance of side effects associated with high concentration of antifungal drugs. Notably, antibiofilm potential of lemongrass oil components alone and in combination with fluconazole has been evaluated in C. albicans for the first time.
These combinations successfully overcome the antifungal resistance associated with biofilms. To confirm the practical utility of these combinations, in vivo studies are necessary.
Conflict of Interests
The authors confirm that this article content has no conflict of interest.
Acknowledgements
SPM carried out all experimental work, data acquisition and analysis, literature search, writing and manuscript preparation. SDH and RGB contributed to experiments, Proof reading. SMK was responsible for study concept, designing and coordinating the research, supervising the work and revising the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Douglas LJ. Candida biofilms and their role in infection. Trends microbiol. 2003; 11: 30-36.
- Douglas LJ. Medical importance of biofilms in Candida infections. Rev Iberoam Micol. 2002; 19: 139-143.
- Alem MA, Douglas LJ. Effects of aspirin and other nonsteroidal antiinflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother. 2004; 48: 41-47.
- Baddley JW, Poppas PG. Antifungal combination therapy. Drugs. 2005; 65: 1461-1480.
- Bink A, Kucharikova S, Neirinck B, Vleugels J, Van Dijck P, Cammue BP, Thevissen K. The nonsteroidal anti-inflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J Infect Dis. 2012; 206: 1790-1797.
- Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR, López-Ribot JL. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother. 2002; 46: 3591-3596.
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod. 2014; 62: 250-264.
- Al Yousef SA. Antifungal activity of volatiles from Lemongrass (Cymbopogoncitratus) and Peppermint (Menthapiperita) oils against some respiratory pathogenic species of Aspergillus. Int. J. Curr. Microbiol. 2013; 2: 261-272.
- Devkatte AN, Zore GB, Karuppayil SM. Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Research. 2005; 5: 867-873.
- Farhang V, Amini J, Javadi T, Nazemi J, Ebadollahi A. Chemical composition and antifungal activity of essential oil of Cymbopogon citratus (DC.) Stapf. Against three Phytophthora Species. Greener J Bio Sci. 2013; 3: 292-298.
- Matasyoh JC, Wagara IN, Nakavuma JL. Chemical composition of Cymbopogoncitratus essential oil and its effect on mycotoxigenic Aspergillus species. Afr J Food Sci. 2011; 5: 138-142.
- Raut JS, Shinde RB, Chauhan NM, Karuppayil SM. Phenylpropanoids of plant origin as inhibitors of biofilm formation by Candida albicans. J. Microbiol Biotechnol. 2014; 24: 1216-1225.
- Raut JS, Karuppayil SM. Phytochemicals as Inhibitors of Candida Biofilm. Curr Pharm Des. 2016; 22: 4111-4134.
- Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS Yeast Res. 2015; 15: fov001.
- Bachmann SP, Ramage G, Vande Walle K, Patterson TF, Wickes BL, López- Ribot JL. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrobl Agents Chemother. 2003; 47: 3657-3659.
- Wagner H, Ulrich-Merzenich G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009; 16: 97-110.
- Doke SK, Raut JS, Dhawale S, Karuppayil SM. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J Gen ApplMicrobiol. 2014; 60: 163-168.
- Shinde RB, Chauhan NM, Raut JS, Karuppayil SM. Sensitization of Candida albicans biofilms to various antifungal drugs by cyclosporine A. Annals Clini Microbio and Antimicrob. 2012; 11: 27.
- Shinde RB, Raut JS, Chauhan NM, Karuppayil SM. Chloroquine sensitizes biofilms of Candida albicans to antifungal azoles. Braz J Infect Dis. 2013; 17: 395-400.
- Khan MS, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemother. 2012; 67: 618-621.
- Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Expe Bio Med. 2004; 229: 567-585.
- Zore GB, Thakre AD, Rathod V. Evaluation of anti Candida potential of geranium oil constituents against clinical isolates of Candida albicans differentially sensitive to fluconazole: Inhibition of growth, dimorphism and sensitization. Mycoses. 2011; 54: 99-109.
- Rao A, Zhang Y, Muend S, Rao R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob Agents Chemother. 2010; 54: 5062-5069.
- Cui J, Ren B, Tong Y, Dai H, Zhang L. Synergistic combinations of antifungal and anti-virulence agents to fight against Candida albicans. Virulence. 2015; 6: 362-371.
- Campbell BC, Chan KL, Kim JH. Chemosensitization as a means to augment commercial antifungal agents. Front Microbiol. 2012; 29: 79.