
Research Article
J Bacteriol Mycol. 2018; 5(3): 1071.
Automatic Early Warning System to Detect and Quantify Legionella Species in Cooling Towers
Rodríguez G1*, Solís I1, Jiménez M1, Sabater M1, Martínez MA1, Bedrina B1, Lázaro M1, Ceña S2, Puig A2, Davide D2, Fisac C2 and Rodríguez J2
¹Biótica SL, Scientific Park of University Jaime I, Castellón, Spain
²Idneo Technologies, Barcelona, Spain
*Corresponding author: Rodríguez G, R&D Department, Biótica SL, Scientific Parc of University Jaume I, Campus Rius Sec, Espaitec 2, Ground Floor, Laboratory 2, Castellón, Spain
Received: May 16, 2018; Accepted: June 21, 2018; Published: June 28, 2018
Abstract
Early determination of Legionella spp concentrations is essential to avoid inadequate or unnecessary disinfection treatments. Culture confirmation takes 10 to 12 days, delaying the identification of potential infection sources. Faster, but accurate alternatives are needed. On site measurement of the Legionella spp concentration will greatly facilitate the timely steps and management of potentially risk sources. We developed a rapid method based on the immunomagnetic separation combined with enzyme-immunoassay for the quantitative determination of Legionella spp in water samples (Legipid®). The aim of this work was to adapt this method to develop a completely automated device able to perform on site. Use of this method for Legionella spp quantification as a fully-automated system could provide dramatic improvement in time-to-result to shortening decision-making process. We describe an automated immunosensor including filtering module and disposable reagents cartridges that allows for rapid determining of Legionella levels. Automated filtering module allow for rapid and efficient water samples concentration. Magnetic immuno-beads provide the separation of the whole cell target from the rest of the sample and their concentration. An optical reader provides easily accessible digital readouts of Legionella concentration measurements. The study evaluated this device as a reasonable approach for Legionella quantification and could produce results in as few as 2 hours with no downstream workup. Performance parameter was also comparable (sensitivity 100%; specificity 92.6%; accuracy 96.7%). The performance of the completely automated analyser allows on-site analysis of Legionella levels without hands-on steps prone to human error.
Keywords: Legionella; Automation; Immunosensing; On-Site; Prevention
Introduction
Legionnaires’ disease (LD) is an environmental worldwide disease comprising pneumonia symptoms ranging from slight fever to lung infiltrates and multisystem failure [1]. The majority of infections are caused by strains belonging to different Legionella pneumophila serogroups [2-4]. This pathogen is a thin, rod-like (0.5-0.7 µm of thickness and 2-20 µm of length) aerobic gram-negative bacterium that inhabits a wide variety of naturally occurring and anthropogenic aquatic zones, where it is essentially an obligate intracellular parasite of free-living protozoa and a secondary colonizer of biofilms [3-4]. Manmade water systems with aerosol generation have been identified as potential reservoirs of this bacterium, such as those found in hotels or resorts, hospitals, long-term care facilities, and cruise ships. Transmission to human lungs can occur by inhalation of aerosolized droplets of water contaminated with the bacterium Legionella (bioaerosols). Among others, showerheads, cooling towers, hot tubs, and decorative fountains have been categorized as risk facilities. Thus, the control of Legionella in these settings has been considered the most effective strategy for prevention of LD [5]. LD is a higher public health priority for research and policy development. LD has around 10-15% fatality rate, ranging 5-30%, and many others who survive only do so after extensive hospital treatment, with an average length of hospital stay of 10.3 days. Total cost of all hospitalizations is over $433,000,000 in United States and $1,359,000,000 in Europe, with a total cost per patient exceeding $24,000–$34,000 [6-8].
Bioaerosols from cooling towers are often suspected to cause community-acquired LD outbreaks. Cooling towers are designed to remove heat from a building or facility by spraying water down through the tower. To prevent and control Legionella contamination in cooling towers, maintenance actions should focus on lowemission cleaning procedures of cooling towers combined with control measurements of water and air samples. An inadequate water management program, dense biofilm within the cooling towers, and high ambient temperatures can promote Legionella spp. proliferation. Management and control strategies should be supported by an improved Legionella detection method that provides reliable, rapid and valuable information related to the public health risk. Moreover, procedures allowing rapid detection and risk assessment in the potential sources of infection, such as cooling towers, are essential for adequate public health measures.
Considerable effort has been focused on the determination of Legionella spp because species of Legionella other than Legionella pneumophila are also causing health issues. The traditional method for Legionella detection in environmental samples is based on cultivation in selective artificial media [9,10]. Although recovery of a bacterial isolate by culture is the standard for identification of Legionella in the environment, well-known limitations of this method could compromise their utility in preventive or rapid control action. Among these drawbacks, we can highlight (a) long time to confirm results (from 2 to 28 days are required), (b) changes on environmental water samples have been identified during their transport to laboratory, which might take up to 1-2 days, (c) likely presence of viable and infective but no-cultivable cells, (d) poor sensitivity, and (e) a rate of inconclusive results up to 20% by interfering microbiota [11,12]. In this scenario, routine testing based on rapid Legionella detection method could be combined with risk assessment and control measures. The use of alternative not-growth based methods to detecting or quantifying Legionella in water samples may permit to complement current epidemiological purposes of the culture method enabling a prevention strategy based on a comprehensive risk assessment. PCR or PMA-PCR are very sensible but they are inappropriate methods for discriminating between live and dead cells, being unreliable to detect Legionella for regulatory purposes [13,14]. Moreover, the lack of correspondence between the results of this PCR techniques and the culture method makes difficult the understanding of the result and its application to take timely steps on the risk facilities.
Current shortcomings in the quantification of Legionella have been reported as a barrier to Legionella control [15], encouraging the development and approval of novel rapid test methods for quantifying live Legionella in water samples. In this context, a method based on immunomagnetic separation (IMS) for Legionella spp monitoring based on the use of anti-Legionella antibodies immobilized on magnetic beads have proved its efficacy [16]. IMS combines specific, whole-cell antibody recognition with magnetic bead-based purification for bacterial concentration. This method has proved to be more sensitive than the culture method, and provides the possibility to selectively quantify viable Legionella cells with an established equivalence between the results using this technique and those obtained using culture results [17]. This test has already been evaluated by Public Health laboratories by comparing this test method with q-PCR and conventional culture [18]. We developed a rapid method based on the immuno-magnetic separation combined with enzyme-immunoassay for the quantitative determination of Legionella spp in water samples (Legipid®). The aim of this work was to adapt this method to develop a completely automated device able to perform on site.
Materials and Methods
Legipid® test method
Legipid® is a test based on immunomagnetic separation (IMS) by anti-Legionella immuno-modified magnetic beads, combined with an enzyme-linked colorimetric detection for a rapid 1 h test. This IMS method is certified by the Research Institute of the Association of Official Analytical Chemist (AOAC-RI). Briefly, original water sample is concentrated by filtration or similar, and this prepared sample is eluted and dispensed into the test cuvette. A suspension of Legionella binding magnetic beads is added. Legionella cells present in the prepared sample will bind to the antibodies immobilized onto the surface of the beads, to form bacteria/bead complexes. Antibodies bind to antigens expressed on the surface of Legionella cells. As these complexes can be separated by a magnet, they can be easily washed and resuspended. Next, complexes are incubated with an enzymeconjugated anti-Legionella antibody to form labelled complexes. After washing steps, the Legionella/magnetic bead complexes are visualized by the colorimetric reaction when enzyme substrates are added. A control (without target) can be tested in parallel in another control cuvette. The results are reported as equivalent colony forming units (CFUeq), i.e. the amount of colony forming units (CFU) that would have been obtained by using the culture method in absence of interfering microbiota and being all Legionella cells available to the antibodies.
Automated analyzer
The analyzer includes a fluidic circuit that performs the following functionalities: sample intake, sample filtering, filter elution, reagents pipetting, liquid waste disposal and circuit sterilizing and cleaning. This fluidic circuit is composed of: two peristaltic pumps, one membrane pump, 5 electro valves, 3 bottles for liquid consumables, an intermediate measurement tank, a buffering manifold, a level sensor, a tube sensor for liquid presence, and an automated coupling system for disposable filters (Figure 1). The automated analyzer includes modules in which discrete aspects of the complete Legionella assay are performed using a plurality of reagents contained in different wells on a disposable polypropylene cartridge (Figure 2). The analyzer has bar code readers so that the instrument can identify each cartridge from the bar code. Then, information regarding all the reagents is specified on the cartridge. All the cartridges (up to 10) are loaded into a refrigerated module at 5±3°C until ready to use.
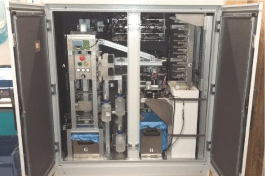
Figure 1: Automated analyzer consisting of: A) Filtering module, B) Elution
and washing zone, C) Cartridge dispenser, D) Fridge, E) Analytical module,
F) Telescopic handler, G) Container of used filters, H) Container of used
cartridges, I) Electric control panel.

Figure 2: Reagents cartridge: A) Empty cartridge, B) Filled and sealed
cartridge, C) Cartridge already used by the analyzer.
Water was collected by installing an external pump. The inlet pressure has to be between 0y 500mBar. Quality of the inlet water was specified in terms of suspension solids content below the 200mg/ mL. The analyzer includes a module for automatically filtering an original water sample, and eluting the concentrated sample on a well in a cartridge dispensed in the analytical module. The original water sample to be collected and filtered is extracted from a bypass loop system by using a peristaltic pump. The dispenser is made of one distribution rail. The rail is positioned vertically. The filters are inserted at the top of the rail and come out at the bottom. The rail is activated by a single servomotor and can contain up to 10 filters. A mechanism enables the dispenser to free one filter at a time. At the bottom of the rail, there is a linear guide with two slides that move in opposite directions, actuated by the same spindle. The dispensed filter is deposited in a holder. The spindle is driven by a DC motor and has two limit switches. The module has a DC motor coupled with an eccentric that will be in charge of making the filter vibrate to release the material retained, including Legionella, after the passage of the volume of water. The analyzer uses a telescopic handler which takes a cartridge from the fridge and then loads it in the analytical module. Nine milliliter-sample loop (Figure 3), transports the concentrated sample from the filtering module to the corresponding well in the cartridge on the analytical module. This analytical module (Figure 4), is integrated by a slide on a dual-axis system (spindle type) driven by stepper motors at the ends: i) X axis is the path with the different positions of the reagents and loading/unloading and ii) Z axis with the purpose of making the vertical movement to introduce the transfer (loading/unloading) pipette inside the wells of a cartridge. To position and fix the cartridge, the motor will activate and once the system has the cartridge fixed correctly, proceed with the analysis with the corresponding analysis positions and the pipetting movements. This same motor is responsible for the movement of the magnets, with the help of springs, to perform the analysis.
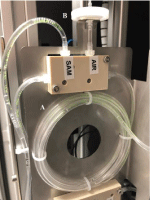
Figure 3: Nine milliliter-sample loop consisting of: A) tygon tubing loop; B)
PTFE Filter membrane pore size 0.2μm.

Figure 4: Analytical module consisting of: A) photometer, B) cartridge holder,
C) Magnets platform, D) Magnet (four magnets per platform).
In the analytical module the analyzer operates through the steps of incubating the elute with immunomagnetic beads at 25±2°C for 15 minutes, performing the magnetic separation procedure, washing of the complexes Legionella-magnetic beads, incubating them with an enzyme conjugated antibody, washing of the complexes enzyme conjugated antibody-Legionella-magnetic beads, adding the substrates of the enzyme, developing colorimetric reaction, separating the complexes from the supernatant, and determining the presence and quantity of Legionella spp by photometric measurement of the supernatant in an integrated photometer with a reading cuvette. Finally, washing of the sample circuit and the reading cuvette and data processing, e.g., concentration conversion, are also operated. Both filter and cartridge used in a reaction are discarded in the corresponding container. The analyzer returns to zero motion after each cycle and ready to do the following analysis. Automated analyzer can be interfaced to the user information system so that after subsequent release of the result, it is automatically transmitted to the user record; this eliminates the need for manual entry of the result in the computer. This is not only time-efficient, but it is also useful for preventing transcription errors during manual entry of the result in the information system.
Response curve
Complete analytical system was tested initially to get the responsecurve of the automated method by measuring the relationship between the transmittance at 455nm obtained by the analyzer and the target concentration in different samples of reference materials having known values tested by the Legipid® test method (certification no. 111101 AOAC-RI). Five levels with five replicate test portions (twenty-five assays) per level of water artificial samples were prepared covering the whole range of interest by spiking Legionella pneumophila serogroup 1 ATCC33152 (Bioréférence, Eurofins, France). The obtained calibration curve was introduced in the software of the analyzer.
Comparison
Testing was performed by comparing the automated method to Legipid® method for enumeration of Legionella spp in cooling tower water samples. Naturally contaminated water samples were tested. For each run one negative control was always conducted. Water samples (a total of 60) of 1 L were collected by the analyzer from a cooling tower in Valencia (Spain). Samples were immediately processed by the analyzer.
Results and Discussion
Response curve
Results are summarized in table 1. The relationship between transmittance at 455nm for the ULISENS unit and the target concentration measured by Legipid® method was calculated as: Where y = CFUeq/volume examined and x= transmittance
CFUeq/vol. examined
ULISENS result by replicate no., transmitance/portion tested
1
2
3
4
5
Transmítanme,
CFUeq/vol. examined log10
mean
340000
0,02
0,02
0,03
0,03
0,03
0,02
5,53
20500
0,31
0,30
0,35
0,33
0,30
0,32
4,31
3980
0,59
0,62
0,61
0,61
0,57
0,60
3,60
175
0,78
0,79
0,78
0,77
0,78
0,78
2,24
53
0,92
0,87
0,91
0,92
0,90
0,90
1,72
Table 1: Relationship between the signal of ULISENS analyzer and concentration of Legionella spp.
Comparison
A total of 60 water samples were assayed by the two techniques (Legipid® method and ULISENS method). Of these, 35 (58.3%) were recorded as positive by at least one method. Legionella spp was detected by Legipid® in 33 (94.3%) of these 35 samples. The proportion of samples positive by Legipid® or ULISENS method was not significantly different. Of the 35 ULISENS method-positive samples, 33 were also positive by Legipid®, leaving 2 (5.7%) discrepant samples positive by the ULISENS method alone. The performance of the ULISENS method was found to be comparable to the Legipid® method. Presentation of the results from the two tests is shown in Table 2.
ULISENS method
Legipid® method
Positive
Negative
Total
Positive
33
0
33
Negative
2
25
27
Total
35
25
60
Table 2: Agreement among ULISENS and Legipid® results.
Data were also examined from the point of view of decisions dependent on levels of action and alert as defined in European Technical Guidelines [19]. According to European Technical Guidelines action is required for more than 10,000 CFU·L-1 (4.0 log10) in cooling towers. That implies turn the tower off until it is known that the controls are in place and the system is safe. The alert and action levels for the ULISENS method were the same as for Legipid®, because high correspondence between the two methods exists. The two tests would have resulted in identical responses for 90.0 % of comparisons (Table 3). In just two cases Legipid® indicated no action is required while the ULISENS method indicated emergency immediate action. In one of these cases both results (3.5 log10 for Legipid® and 4.1 log10 for ULISENS) were close to the threshold value (4.0 log10). The results proved that ULISENS method is equivalent to the Legipid® reference method for detection of Legionella spp. at tested contamination levels ranging from low (2-3 log10 CFUeq/volume examined, medium (3-4 log10 CFUeq/volume examined), and high (greater than 4 log10 CFUeq/volume examined) in potable water and industrial water matrixes.
Legionella spp
ULISENS method no.
Action
Alert
Satisfactory
Total
=104 CFUeq l-1
=103 CFUeq l-1
<103 CFUeq l-1
Legipid® no.
Action
=104 CFUeq l-1
16
1
0
17
Alert
=103 CFUeq l-1
1
5
0
6
Satisfactory
<103 CFUeq l-1
1
3
33
37
Total
18
9
33
60
Table 3: Comparison of action/alert levels using immunomagnetic separation based methods (IMS) Legipid® and ULISENS for Legionella spp determination.
Quantitative data presented by the analyzer in the form of equivalent colony forming units per liter water (CFU L-1) could form the basis for remedial action, if any, according to different published guidelines used to manage the risk of contracting Legionella in cooling towers, thereby protecting public health. It can take less than 2 hours for the full diagnosis and report to become available using the automated IMS method, thereby reducing the likelihood of exposure to Legionella in contaminated cooling towers following delays in taking preventative actions (surveillance, cleaning and disinfection). The need of sampling protocol involving preservation procedures and transport conditions is avoided because the actual sample is taken on-site to be immediately analyzed, from sample collection through sample analysis to result obtention.
Reliable quantifying of Legionella could be achieved in comparison with other techniques. Culture enumeration can underestimate the risk of Legionella due to, among others issues, slow growth rate of Legionella in a plate, overgrowing of accompanying organisms, inability to count viable but non-culturable (VBNC) organisms, presence of vesicles containing Legionella expelled from protozoa, or loss of cultivability during sample holding time prior culturing. For some samples containing PCR inhibitors, high quantification limits do not allow the quantification of the target by this technique in complex waters [20]. Automated analyzer provided repetitive measurements without any processes involving human manipulation. Data could be hosted in the cloud, remaining available for further studies. Target cells are separated from debris or other cells by immunomagnetic separation as a preparatory step, prior to the detection, even though some of the samples presented dirtiness that made handling difficult. Thus, the automated IMS method reduced the likelihood of inconclusive results in Legionella testing. Results indicated that the ULISENS method could be a more reliable option for routine testing, particularly in the analysis of water samples with high levels of contamination. It could become an important component in the establishment of regular monitoring programs on the basis of reliable and fast on-site Legionella quantification.
Conclusion
Automatic Legionella detection eliminates human errors inherent in the manual methods. Robotic assay handling reduces the variability of the results, removes the risks of miscounts, mid-test contamination and incorrect data entry, leading to fewer inconclusive results and supporting timely steps on the risk facility. Timely steps ultimately mean more uptime, less delay and fewer re-tests. Rapid IMS-based test for environmental Legionella monitoring can produce reliable on-site results within 2 hours, and monitored installations can be analyzed for safety in significantly less time than traditional methods. Such quick results allow maintenance personnel to respond immediately to contamination events, finding and eliminating bugs before the bacterium spread. Owners can rest assured their processes are safe for public health. Changes on both concentration and physiological status of Legionella can occur over short and unpredictable periods of time [21,22]. As the concentration of free Legionella cells in the water can fluctuate, fast and reliable methods for Legionella monitoring are needed. By eliminating redundant tests and hastening shipments, automatic quantification can helps owners achieve the highest possible control on site. The performance of the ULISENS systems was found to be comparable to the reference Legipid® method. The environmental testing for Legionella has been identified as a key challenge by all the potential customers. It is generally recognized that the main analytical issue is to separate the target from the rest of the sample. Among the cultivation-independent methods, those using magnetic immuno-beads provide the separation of the whole cell target from the rest of the sample and their concentration. This attachment is mediated by antibodies immobilized onto the surface of the beads and the antigens expressed on the surface of Legionella cells. So that, this binding depends on the cell envelop integrity and it is independent on the growth capacity of the cell, often low in the wild Legionella strains. That provides the analytical result with a relevant meaning from the point of view of public health protection. Cooling towers (CTs) are a leading source of outbreaks of Legionnaires’ disease (LD), so that proper maintenance of CTs is vital for the prevention of LD. The aim of this study was to develop a completely automated device able to provide onsite information useful for this purpose.
Acknowledgments
The authors would like to thank the European Union’s Horizon 2020 research and innovation programme for the financial support under grant agreement No 726499.
References
- Swartz MN. Clinical Aspects of Legionnaires´ Disease. Ann. Intern. Med. 1979; 90: 492-495.
- Lück C. Legionella-a case for culture. Indian J Med Res. 2010; 131: 736-738.
- Fields BS. The social life of Legionellae. In: Marre R, Abu Kwaik Y, Bartlett C, Cianciotto NP, Fields BS, Frosch M, et al., editors. Legionella. Washington DC: ASM Press; 2002: 135-142.
- Valster RM, Wullings BA, Bakker G, Smidt H, van der Kooij D. Free-Living Protozoa in Two Unchlorinated Drinking Water Supplies, Identified by Phylogenic Analysis of 18S rRNA Gene Sequences. Appl. Environ. Microbiol. 2009; 75: 4736-4746.f
- Garrison LE, Kunz JM, Cooley LA. Vital signs: Deficiencies in environmental control identified in outbreaks of Legionnaires’ disease - North America. 2000-2014. MMWR Morb Mortal Wkly Rep. 2016; 65: 576–584.
- Pierre DM, Baron J, Yu VL, Stout JE. Diagnostic testing for Legionnaires’ disease. Annals of Clinical Microbiology and Antimicrobials. 2017; 16: 59.
- Farnham A, Alleyne L, Cimini D, Balter S. Legionnaires’ disease Incidence and Risk Factors, New York, New York, USA. 2002–2011. Emerging Infectious Diseases. 2014; 20: 1795-1802.
- Beauté J, On behalf of the European. Legionnaires’ disease Surveillance Network. Legionnaires’ disease in Europe. 2011 to 2015. Eurosurveillance. 2017; 22: 30566.
- International Organization for Standardization. 1998. ISO 11731:1998. Water quality-detection and enumeration of Legionella. International Organization for Standardization, Geneva, Switzerland.
- International Organization for Standardization. 2004. ISO 11731-2:2004. Water quality - Detection and enumeration of Legionella - Part 2: Direct membrane filtration method for waters with low bacterial counts. International Organization for Standardization, Geneva, Switzerland.
- Lee TC, Vickers RM, Yu VL, Wagener MM. Growth of 28 Legionella sppecies culture media: A comparative study. J. Clin. Microbiol. 1993; 31: 2764–2768.
- Garcia MT, Jones S, Pelaz C, Millar RD, Abu Kwaik Y. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 2007; 9: 1267-1277.
- Benowitz Isaac, et al. Rapid Identification of a Cooling Tower-Associated Legionnaires’ Disease Outbreak Supported by Polymerase Chain Reaction Testing of Environmental Samples, New York City. 2014-2015. Journal of Environmental Health. 2018; 80: 8-12.
- Taylor MJ, Bentham RH, Ross KE. Limitations of Using Propidium Monoazide with qPCR to Discriminate between Live and Dead Legionella in Biofilm Samples. Microbiol. Insights. 2014; 7: 15-24.
- Peter A, Thompson KC, Routledge EJ. Barriers to effective Legionella control in a changing world: A practitioner’s view. Environmental Technology Reviews. 2017; 6: 145-155.
- Bedrina B, Macián S, Catalán V, Solís, I, Fernández-Lafuente R, Baldrich E, Rodríguez G. Fast immunosensing technique to detect Legionella pneumophila in different natural and anthropogenic environments: Assessment and validation. BMC Microbiol. 2013; 13: 88.
- Rodriguez G, Bedrina B, Jiménez M. Method modification of the legipid® legionella fast detection test kit. JAOAC Int. 2014; 97: 1403-1409.
- Díaz-Flores á, Montero JC, Castro FJ, et al. Comparing methods of determining Legionella spp. in complex water matrices. BMC Microbiology. 2015; 15: 91.
- European Technical Guidelines for the Prevention, Control and Investigation of Infections Caused by Legionella Species. 2017.
- Touron-Bodilis A, Pugnard C, Frenkiel-Lebossé H, Hallier-Soulier S. Usefulness of real-time PCR as a complementary tool to the monitoring of Legionella spp. and Legionella pneumophila by culture in industrial cooling systems. J Appl Microbiol. 2011; 111: 499-510.
- Ragull S, Garcia-Nuñez M, Pedro-Botet ML, et al. Legionella pneumophila in Cooling Towers: Fluctuations in Counts, Determination of Genetic Variability by Pulsed-Field Gel Electrophoresis (PFGE), and Persistence of PFGE Patterns. Applied and Environmental Microbiology. 2007; 73: 5382-5384.
- Stout, Janet E, et al. Environmental culturing for Legionella: Can we build a better mouse trap? American Journal of Infection Control. 2010; 38: 341–343.