
Research Article
J Bacteriol Mycol. 2018; 5(4): 1073.
Determination of the Frequency of Lea Antigens, Leb and Anti-Le Antibodies in Individuals Infected or not by Helicobacter Pylori
Rampelotti JC, Bueno EC, Valcarenghi D and Geraldo A*
University of Vale do Itajaí, Brazil
*Corresponding author: Alexandre Geraldo, University of Vale do Itajaí, Uruguai Street, Brazil; Email: alexandregeraldo@univali.br
Received: June 01, 2018; Accepted: July 03, 2018; Published: July 10, 2018
Abstract
Introduction: Helicobacter pylori, has an affinity for the gastric mucosa, and may be associated with blood group systems, among them the Lewis System. The adhesins are one of the virulence factors of H. pylori and studies have indicated as receptors for adhesins certain blood group antigens. Therefore, the objective of this study was to determine the frequency of Lea antigens, Leb antigens and anti-Lex antibodies in individuals infected or not with H. pylori.
Methods: Samples were subjected to phenotype for Lea and Leb antigens and serological tests for determination of H. pylori using IgA / IgG / IgM class antibodies.
Results: Of the 88 individuals surveyed, 18% had Lea antigen, 74% Leb and 8% did not present Lea and Leb antigens in the erythrocytes studied. 54% of the students who had active H. pylori infection expressed the erbocytic Leb antigen.
Discussion: The Le (a-b+) phenotype was the most prevalent with 74%. Individuals who reported having no symptoms had a higher percentage of active infection by H. pylori, with 17%, and were carriers of the Le (a-b+) phenotype.
Conclusion: The group with the highest frequency of active H. pylori infection was the Le (a-b+) phenotype, and this antigen could also be associated with the Leb antigen isomers, thus serving as a receptor for H. pylori in the tissue. In this way, drugs may be developed with a mechanism to prevent the binding of H. pylori to the Lewis System antigens in the gastric cavity during treatment, preventing infection.
Keywords: Lewis Blood Group System; Helicobacter Pylori; Gastritis; Peptic Ulcer; Antigens
Introduction
In the 1970s and 1980s, researchers used silver staining to evaluate biopsies of peptic ulcers and identified curved bacteria that colonized the gastric antrum of hosts. Thus, it was possible to describe the microbiological properties of these bacteria, which were called Campylobacter pylori, which later became known as Helicobacter pylori (H. pylori) [1].
The genus Helicobacter was defined by studies of the composition of ribosomal RNA [2], sequencing and hybridization of the DNA of the bacterium [3]. This genus, along with others (Campylobacter, Arcobacter and Wolinella), constitutes the superfamily VI of gramnegative bacteria [4].
The morphology of H. pylori, observed under optical and electronic microscopy, is homogeneous, presenting a curved or spiral structure, with a smooth surface and rounded, mobile, non-spore and microaerophilic ends. It measures approximately 0.5μm to 1μm in width and 3μm in length, having four to six sheathed unipolar flagellae and terminal bulbs at the smooth ends [5]. The oral-oral, gastrooral and fecal-oral transmission are the most probable among other possibilities, such as the one that suggests that the drinking water is vehicle of the bacterium. This bacterium synthesizes urease, which generates ammonia, damaging the gastric mucosa, since ammonia neutralizes the acidic pH of the stomach, allowing the body to live in the gastric mucosa [6]. It is estimated that about 50% of the world population is infected with H. pylori, and this prevalence is higher in developing countries [7]. In Brazil, the prevalence of H. pylori has been reported among adults and children at rates ranging from 34% to 80% [7-10]. Population studies in regions with poor living conditions have shown that, in general, prevalence rates are characterized by a rapid increase with age from the onset of childhood, reaching a plateau around 80% after 20 years of age [11-13].
Another important point is the zoonotic transmission of the genus Helicobacter, due to the presence of gastric microorganisms with similar morphology in the stomach of several animal species (dogs, cats, pigs, cattle, sheep, birds, ferrets, Guinea pigs, monkeys, mice, hamsters, marmots, foxes, cheetahs, dolphins, beluga whales and others). For this reason domestic animals have been frequently reported as potential sources of Helicobacter spp infection [14].
In the literature there are few epidemiological studies of genetic predisposition for gastric diseases. However, previous studies have shown an association of antigens of Lewis and ABH blood groups with susceptibility to H. pylori colonization [15,16].
The Lewis Blood Group System was discovered in 1946 after the identification of the antigen Le ant antigen in children with fetal and neonatal hemolytic disease (DHFRN). Years later, in 1949, researchers demonstrated the presence of Lewis antigens in saliva and plasma. Thereafter, they demonstrate the loss of these Lewis antigens in vivo. This system is currently composed of six glycolipid and glycoprotein antigens, present in saliva, tear, urine, gastric juices, epithelial tissues, bone marrow, kidneys, lymphocytes, platelets, pancreas, adrenals and skeletal muscle [17].
Among blood groups, Lewis antigens are the only ones not to be synthesized by erythrocytes. They are produced by tissue cells and secreted in organic liquids, and their antigens are mainly found in secretions and plasma [18].
Expression of the Lewis antigens depends on the inherited alleles at two independent locus (secretor [SE] gene) FUT2 and (Lewis [LE] gene) FUT3. These alleles encode separate fucosyltransferases that interact to form Lewis antigens in secretions and fluids. The formation of these antigens occurs through the adsorption of carbohydrates from the plasma. These antigens are widely distributed in human and fluid tissues [15]. The FUT3 enzyme acts on the phenotypic expression of the Lewis System by fucosylation of precursor oligosaccharides (OP) type 1 (Galactose-β1 → 3N-acetylglucosamine-β1-R) and type 2 (Galactose-β1 → 4N-acetylglucosamine-β1 -R). These precursors have a free and non-reduced terminal end to which fucose molecules can be added, and an opposite one, which can be attached to carbohydrates, lipids or proteins [16,19,20,21]. Fucosyltransferase Lewis adds a fucose molecule to the carbon-2 of N-acetyl glucosamine (NacGlu) to generate the Le ant antigen (Galactose-a1 → 3 [Fucose-a1 → 2] N-acetyl glucosamine-R). The secretory fucosyltransferase adds a fucose molecule to the carbon 2 of the terminal galactose of the precursor oligosaccharide, giving rise to H-type 1 antigen (Fucose-a1 → 2Galactose-a1 → 3N-acetylglucosamine-R). Fucosyltransferase Lewis adds another fucose molecule to the carbon-2 of N-acetyl glucosamine, generating the antigen Leb (Fucose-a1 → 2Galactose-a1 → 3 [Fucose-a1 → 2] N-acetyl glucosamine-R) [21]. Therefore, the Le ant antigen has epitopes that may be present in type 1 H, which may cross-react with anti-Lea and anti-H antibodies (Figure 1) [22].
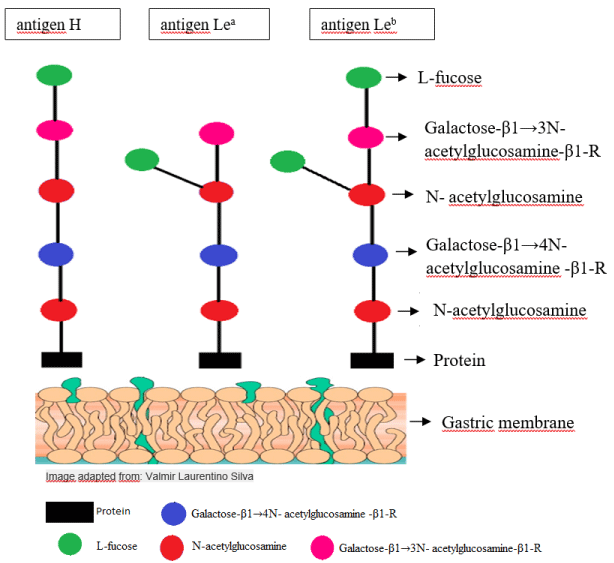
Figure 1: Schematic representation of Lewis antigen synthesis.
When an individual is exposed to allogeneic erythrocytes, blood transfusions, gestations and/or transplants of organs / tissues or grafts, erythrocyte alloimmunization occurs, in which it is an immune response to these allogeneic antigens, resulting in the production of irregular antibodies [23]. These irregular antibodies, also called alloantibodies (except anti-A and anti-B) react against erythrocyte antigens [24].
In 1949, a rare, anti-Lex antibody, which reacts with all Le (a-b+) and Le (a+b-) phenotypes, except for the Le (ab-) phenotype. This antibody is present more frequently in H. pylori-infected individuals [25].
In view of the above, the objective of the present study was to determine the frequency of Lea antigens, Leb erythrocytes and anti-Lex antibodies in individuals infected or not with H. pylori.
Methods
Sampling
After approval by the Ethics and Research Committee on Human Beings of UNIVALI, 1,016,880, the Free and Informed Consent Form was signed, with the completion of a questionnaire that had the objective of capturing data such as: age of the participants, frequency of symptoms, if they had already been treated for H. pylori and had some pathology that weakened the immune system. The samples were collected by venipuncture, using as anticoagulant 1mg/mL ethylene diamine tetra acetic acid di- sodium salt (EDTA) to identify the Lewis phenotype in erythrocytes.
For inclusion criteria in the study participants should be students of the Pharmacy or Biomedicine course, over the age of 18 years. According to the answers of the questionnaire previously applied to the collection, students diagnosed with H. pylori who had been treated for this infection were excluded, also excluding those who were using antibiotics and students with weakened immune systems (HIV seropositivity, and other pathologies related to the immune system reported in the questionnaire applied prior to collection). In addition, individuals who had inconclusive results for the anti-H serologic markers were excluded during the tests H pylori.
In order to classify the frequency of the students’ symptoms, a questionnaire was used in which the frequency of the symptoms was described in: Very, Little, Rare and individuals who never had symptoms. The criteria established for the classification of symptoms related to H. pylori infection were according to the temporality described below: Very (Every day), little (2 to 3 times a week), Rare (1 to 2 times per month) and not for individuals who have never had symptoms. This temporality was established since, H. pylori infection is often asymptomatic and when symptoms occur they represent nonspecific forms of stomach discomfort [26], and pain is established as a criterion of stomach discomfort.
Determination of Lea and Leb antigens
From the 5% red cell suspension, 25μl of the suspension was added to a tube for 50μl of proteolytic enzyme, and incubated at room temperature for 10 minutes. Washing was performed with 0.9% NaCl, after centrifugation for 45 seconds at the rotation of 2336g. After washing, 1 drop of Anti-Lea or Anti-Le an antiserum (Fresenius Kabi®) was added, the tubes homogenized and incubated at 2 to 8° C for 15 minutes. The tubes were then centrifuged for 15 seconds at a rotation of 2336g and read. The presence of agglutination indicated that the erythrocytes had the corresponding antigen.
Determination of IgG/ IgA/IgM class antibodies
The H. pylori antigen adhered to a solid support (Serion ELISA plate) was incubated with the individual’s plasma in the search for antibodies against the antigen. In the case of a positive sample, that is, a patient with serum antibodies, antigen-antibody binding occurs, later detected by the addition of antibody directed against immunoglobulins bound to peroxidase. This antibody bound to the enzyme is called conjugate and when adding to this product the appropriate substrate, the wells where the antigen-antibody reaction occurred have a coloration. The reading was performed from the analysis of the optical density (OD) at 405nm against the blank of the substrate; reference wavelength between 620nm and 690nm [27]. From the results, the following parameters were identified: Active infection (IgM and IgA reagent markers) and previous contact (IgG reagent markers).
Irregular antibody screening
For the detection of irregular antibodies (PAI), two tubes with the numbers I and II were identified, and two drops of serum or plasma of the sample and one drop of OI red blood cells (Triacel®) were added to tube I and in tube II a drop of OII red blood cell suspension (Triacel®). The tubes were homogenized and centrifuged for 15 seconds in a rotation of 2336g. The reading was performed, visualizing the absence of agglutination. BioPeG® 15% potentiating reagent (Fresenius Kabi®) was then added, the tubes homogenized, incubated in a 37°C water bath for 15 minutes and then washed 3 times with 0.9% NaCl solution. To this product was added two drops of Coombs anti-IgG Serum (Bio-Rad®), the tubes were centrifuged for 15 seconds in a rotation of 2336g, and the reading was performed visualizing the absence of agglutination in both. To confirm the result, a drop of red cells was added to Coombs Control (Bio-Rad®) in all tubes, which were centrifuged for 15 seconds in 2336g and then read.
Data analysis
Using the Excel® worksheet, the mean and standard deviation of the individuals’ ages were calculated, as well as the prevalence between the female and male sexes and the frequency of the phenotypes of the Lewis blood group system. In the spreadsheet the percentage of individuals in active infection and previous contact was sectioned according to the frequency of the symptoms described in the questionnaire, thus establishing the prevalence of frequency of symptoms with the Lewis phenotype.
Results
All the students surveyed presented negative Irregular Antibody Search, in this way the anti-Lex was not detected. However, two individuals were excluded because they presented inconclusive serological tests for the determination of anti-H antibodies H pylori. Thus, the number of students for this study was 88 individuals.
Analyzing the data obtained, of the 88 students evaluated, 27% (24/88) were in the Pharmacy course and 73% (64/88) of the Biomedicine course. Among these, the percentage of males was 15% (13/88) and females 85% (75/88), with a mean age of 23 ± 6 years.
Leb antigen (Le (a-b+) phenotype) and 8% (7%) were the Lea antigen (Le (a+b-) phenotype, 18% (16/88), 74% (65/88) did not present the Lea and Leb antigens (Le (a-b-) phenotype).
According to the results obtained in serology for anti-H. pylori high percentages of reagent individuals were observed for the combinations of antibodies tested (IgA, IgM and IgG). Table 1 shows that the higher frequency of individuals with the active infection had the Le (a-b-) phenotype presenting 54%. In the Le (a+b-) phenotype, the students with active infection presented 12% and in the Le (a-b-) phenotype presented 7% with active infection. Of the individuals who had previous contact, 11% had the Le (a-b+), 3% Le (a+b-) and 1% had the Le (a-b-) phenotype (Table 1).
Results
Le(a-b+)
Le(a+b-)
Le(a-b-)
Active Infection
54
12
7
Contact Us
11
3
1
Absolute number of Lewis phenotypes
65
15
8
Table 1: Absolute number of individuals with Active Infection and Pre-contact according to the Lewis Group Blood System phenotype.
Performed for individuals who had prior contact. Observing Table 1 it is possible to observe that the highest percentages of presence of symptoms are present in the Le (a-b+) phenotype. However, it is also possible to observe that 17.04% of this phenotype did not show any symptoms of pain. In those individuals who only had prior contact (IgG-reactive markers), the group that reported low frequency of symptoms had the highest percentage for the Le (a-b +) phenotype of 5.7% (Table 2).
Active Infection
Frequency of Lewis phenotypes
Symptoms
%Le(a-b+)
%Le(a+b-)
%Le(a-b-)
Very common
14.70%
3.4%
2.3%
Less common
14.7%
3.4%
2.2%
Rare
14.7%
3.4%
0%
No
17.0%
3.4%
3.4%
Contact Us
Frequency of Lewis phenotypes
Symptoms
%Le(a-b+)
%Le(a+b-)
%Le(a-b-)
Very common
2.3%
0%
0%
Less common
5.7%
1.1%
0%
Rare
2.3%
0%
0%
No
2.3%
2.2%
0%
Table 2: Frequency of symptoms in individuals with Active Infection and Precontact with phenotypes of the Lewis Blood Group System.
Discussion
There is a paucity of epidemiological studies of genetic predisposition for gastric diseases related to H. pylori. However, previous studies have shown an association of the Lewis blood group antigens with the susceptibility to H. pylori colonization. In this study, the authors indicate that the probable H. pylori receptors in the gastric epithelium are gastric mucin carbohydrates, which may have a primordial function in the protection of the gastric mucosa. Lewis blood group antigens are part of the gastric mucin composition and have been implicated as H. pylori receptors in the gastric mucosa [28].
In this study, individuals who reported having no symptoms had a higher percentage of active infection by H. pylori, with 17%, and were carriers of the Le (a-b+) phenotype. Rauws and Tytgat (1990) found that H. pylori infection is often asymptomatic and when symptoms occur they represent nonspecific forms of stomach discomfort [26]. In the present study, we found percentages of individuals with active infection who presented some frequency of symptoms (14.7%) in a greater number in individuals who had Leb antigen than those who did not have this antigen (0% to 3, 40%). However, this higher percentage of symptom frequency may also be related to the higher frequency of secretory individuals found in the study population, since 76.1% of the individuals presented the Leb antigen.
In the Brazilian Caucasian population, the phenotype Le (ab-) presented 7.79%, whereas in the Le (a+b+) phenotype, Caucasians and Negroides were found to be 1.20% and 0.97%, respectively [29]. Phenotype is more common in other populations, such as Japanese, Thai, and Polynesian, with a frequency of up to 25% being reported in Taiwanese Chinese [30]. Beiguelman (2003) reports that the frequency of these phenotypes varies among different populations. In Caucasians, the frequency of the Le (a+b-) phenotype is 22%, Le (a-b+) 72% and Le (a-b-) 6%. In Japanese, the Le (a-b-) phenotype is less than 2% whereas in Negroid the frequency varies between 20 and 25% (18). In the present study, the Le (a-b+) phenotype presented 74%, the same prevalence as the Beiguelman study (2003). Thus, the results obtained did not show divergences in relation to the Caucasian population, since in the literature the predominance for this population is of the Le (a-b+) phenotype, and in this study the individuals who participated in the research were in their great majority to Caucasians, only two individuals were Afro-descendants. In the study by Novaretti et al. (2000), Brazilian Caucasians presented 75.42% for the Le (a-b+) phenotype, which agrees with the percentage found here.
The reason for an individual to develop gastric diseases may be related to the presence of H. pylori virulence factors such as CagA (cytotoxin A associated gene), VacA (vacuolar A cytotoxin gene) and BabA (antigen binding adhesin of blood group) in bacterial strains that favor bacterial colonization from interactions with host genetic markers such as ABH and Lewis blood group antigens [31]. The BabA gene encodes an adhesin mediating the binding with Le ant antigens expressed in gastric epithelial cells. The relationship between BabA and Le ant antigens is the best characterized adhesin-receptor interaction [6].
In relation to Lewis blood group antigens and their interaction with pathogens, studies with Candida albicans (strain 2346) have demonstrated the expression of an adhesin that binds to fucose, the immunodominant sugar of the Lewis System antigens [32]. Thus, it is possible to notice that the Lewis System antigens are not correlated only with H. pylori, but also with other pathogens such as fungi. One study reported the importance of the Lewis system in leprosy, where possible bacillus receptors could be protected or covered by Lewis and secretory gene products, preventing or facilitating the adhesion of Mycobacterium leprae to the cell surface [33].
Lewis system phenotypes in erythrocytes may be altered in cases of disease and other conditions such as in immunocompromised patients. This can be due to the fact that Lewis system antigens are adsorbed to red blood cells through carbohydrates present in plasma [34]. This phenomenon was observed in patients with cancers of the pancreas, stomach, bile duct, colon, breast and bladder [34]. In addition, there are also indications that, in pregnant women, alcoholic cirrhosis, alcoholic pancreatitis, viral and parasitic infections, may alter Lewis phenotypes [34]. Thus, the erythrocyte phenotyping of the Lewis system alone is not sufficient to determine and characterize the expression of the antigens of this system in the gastrointestinal tract. Thus, FUT3 genotyping constitutes a more reliable strategy for inferring Lewis phenotypes both in erythrocytes and in other tissues and secretions [34].
During the IPA, all the individuals presented negative results. In this way, no individual possessed anti-Lex. According to the study by Zanelli, et al. (2013) irregular antibodies are found in 0.3-38% of the population depending on the group studied, so the negative results for the EPI of this study are related to the population surveyed [24].
A study related to Lewis antigens demonstrated that the antigens with the highest relation to the H. pylori strain are Le? and Le?, and that 80% of the strains were detected along with the Ley and Lex [32] antigens. Another study using three Serodot, Western blot and ELISA serological methods indicated that 20 of the 34 strains (59%) expressed Lex antigens [35].
The Lewis system is composed of six antigens: Lea, Leb, Leab, Leb, LebH, ALeb and BLeb, Ley and Lex being isomers of Lea and Leb [17]. The fact that Lex and Ley are Lean and Leb isomers confers a great relationship between them, so the results can be considered relevant, although no statistical analysis has been performed. This is because it depends on the population studied, as in the study by Simoons-Smit, et al. (1996), in which the dominant population was Chinese, resulting in a higher prevalence of Lex. In this study, all are of Brazilian nationality, occurring a prevalence of Leb according to the results found, not being evaluated the isomeria of the same as in the case of Law [36].
The Ley antigen is a difucosylated tetra saccharide derived from the type 2 precursor chain, a Leiden antigen positional isomer and a fucosylated Lex derivative. Its difference from the Lex antigen is due only to the addition of the terminal residues 1,2 fucose [37]. Fucosylation of the precursor oligosaccharide (OP) type 1 allows the expression of Lea and Leb antigens, which can be expressed in endodermal tissues. On the other hand, fucosylation of type 2 OP leads to the production of Lex and Ley antigens in tissues of mesodermal origin such as vascular endothelium [19]. Therefore, the expression of these antigens depends on the type of oligosaccharide available in the tissue [38].
Daniels, described that Lex and Ley are Lea and Leb type 2 isomers respectively, but are not really blood group antigens because they are not able to detect erythrocytes [19]. Thus, due to positional isomerism, the antigens described in this study, such as Lea and Leb, may still be masked, due to the structure similar to that of Lex and Ley because they were not detected in erythrocytes, Lewis phenotyping only described the two antigens that are adsorbed on erythrocytes and were investigated in this study, genotyping could be confirming isomerism, but it was not possible to perform this technique. In the study of Hynes and Moran (2000), the percentage found in H. pylori strains was of Lex antigens, because this is Lea and Leb isomer, explains the result of the higher percentage in students with Le (a-b +) phenotype [35].
An important factor when comparing the sensitivity and specificity between the serological tests and the invasive methods for detecting H. pylori is that the latter have a lower rate of false positive results, which has not been evaluated in the serological study [27]. On the other hand, the study by invasive methods may present false negative results due to the inhomogeneous distribution of the bacterium in the stomach [39,40].
In serology for anti-H. pylori, the sensitivity and specificity rates reported by the manufacturer for the IgG antibody were 93.8% sensitivity and 94.1% specificity, whereas IgM had 100% sensitivity and 77.8% specificity and IgA had 79.8% sensitivity and 93.2% specificity [27].
Sudraba, et al. (2011) compared several diagnostic methods for H. pylori, with sensitivity and specificity as parameters. In the rapid urease test, sensitivity was 96% and specificity was 100%; however, sensitivity was 96% and specificity of 50%, with large numbers of false positives occurring [41]. Serological tests are not gold standard for H. pylori screening in the gastric mucosa, but because they are highly sensitive they could be used to screen individuals for endoscopy.
In the studied population, the individuals attended the same environments and used restaurants, snack bars and classrooms of the institution where the research was carried out. The transmission of H. pylori is very broad, it is understood that there is a predisposition of the individuals to be infected, or for the food, because they attend restaurants and cafeterias of the institution, or by the very coexistence of the students. This explains the high percentage of individuals with active H. pylori infection.
Conclusion
The group that presented the highest frequency of infection by the bacterium was the one with the Le (a-b+) phenotype, since the majority of the study population was Caucasian, and for this population there is a predominance of this phenotype. Lea and Leb antigens have isomeria with Lex and Ley, thus being a second alternative for future research in order to obtain information if there is a relation of the binding to the antigens or their isomerism. It is important to emphasize that this study was carried out through erythrocyte antigens and that it is extremely important to perform histological studies between H. pylori and Lewis system antigens.
Another important factor would be the study of the binding of H. pylori isomers in the tissue. Future drugs could be developed with the mechanism to prevent the binding of H. pylori to Lewis system antigens in the gastric cavity or mainly to the immunodominant carbohydrate (fucose). Thus, future treatments would be directed towards the prevention of recontamination during treatment and not only for the death of H. pylori, since this future treatment would only prevent the binding of H. pylori. Thus, it would ease recurrence during treatment, reducing the amount of medication used by the patient and maintaining a balance of the microbiota, since the treatments are not specific for H. pylori, and end up destroying the patient’s bacterial flora, causing an imbalance which may lead to a decrease in the immunity of the individual.
Serology for anti-H. pylori, because it has high sensitivity could be used in the form of screening before endoscopy (invasive method), since it could define if the individual had contact and acquired memory.
References
- Warren RJ, Marshall B. Unidentified curbed bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983; 1: 1273-1275.
- Romaniuk PJ, et al. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J. Bacteriol. 1987; 169: 2137-2141.
- Goodwin CS, et al. Campylobacter pylori become Helicobacter pylori. J. Bacteriol. 1989; 2: 1019-1020.
- Vandamme P, et al. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: Emendation of generic descriptions and proposal of Arcobacter gene. Nov. Int. J. Syst. Bacteriol. 1991; 41: 88-103.
- Ladeira MSP, Salvadori DMF, Rodrigues MAM. Biopathology of Helicobacter pylori. Brazilian Journal of Pathology and Laboratory Medicine. Rio de Janeiro. 2003; 39: 335-342.
- Barbosa JA, Schinonni MI. Helicobacter pylori: Association with gastric cancer and new findings on virulence factors. Journal of Medical and Biological Sciences, Salvador. 2011; 10: 254-262.
- Carvalho AST, Queiroz DMM, Mendez EN, Rocha GA, Penna EJ. Diagnosis and distribution of Helicobacter pylori: In the gastric mucosa of symptomatic children. Journal of Pediatrics. Rio de Janeiro. 1991; 24: 163-166.
- Coelho LG, Das SS, Karim QN, Walker MM, Queiroz DMM, et al. Campylobacter pyloridis in the upper gastrointestinal tract: A Brazilian study. Arq Gastroenterol. 1987; 24: 5-9.
- Oliveira AMR, Queiroz DMM, Rocha GA, Mendes EN. Seroprevalence of Helicobacter pylori infection in children of low socioeconomic level in Belo Horizonte, Brazil. Am J Gastroenterol. 1994; 89: 2201-2204.
- Rocha GA, Queiroz DMM, Mendes EN, Oliveira AM, Moura SB, Silva RJ. Source of Helicobacter pylori infection: Studies in abattoir workers and pigs. Am J Gastroenterol. 1992; 87: 1525.
- Malaty HM. Epidemiology of Helicobacter pylori Best Pract & Res Clin Gastroenterol. 2007; 21: 205-214.
- Graham DY, Yamaoka Y, Malaty HM. Thoughts about populations with unexpected low prevalences of Helicobacter pylori infection Trans. R Soc Trop Med Hyg. 2007; 101: 849-851.
- Logan RPH, Walker MM. ABC of the upper gastrointestinal tract: Epidemiology and diagnosis of Helicobacter pylori Brit. Med. J. 2001; 323: 920-922.
- Carvalho GD, Pinto PSA, Viloria MIV, Nero LA. Aspectos zoonóticos de Helicobacter spp. Biosci. J. Uberlândia. 2008; 24: 121-130.
- Combs MR. Immunohematology. Journal of Blood Group Serology and Education. 2009; 25: 112-118.
- Schenkel-Brunner H. Lewis System and the antigens Lex and Ley, Alexandra Salvini-Plawen. Chemical and Biochemical Basis of Antigen Specificity. 2th Vienna, Austria, Editora: Springer-Verlag. 2000: 184-224.
- Girello AL, Kuhn TIBB. Fundamentos da imunohematologia eritrocitária. São Paulo, Editora: Senac, 3a edição. 2011.
- Beiguelman B. Os Sistemas Sanguíneos Eritrocitários. Editora FUNPEC, Ribeirão Preto- SP, 3ª Edição. 2003: 234.
- Daniels G. ABO, Hh, and Lewis blood group systems. Human blood groups. Cambridge University Press. 1995.
- Henry SM. Phenotyping for Lewis and histo-blood group antigens secretor. Immunohematology. 1996; 12: 51-56.
- Oriol R. ABO Hh, Lewis and Secretion: serology, genetics and tissue distribution. In: Cartron JP, Rouger P. Blood cell biochemistry: molecular basis of human blood group antigens. New York: Plenum. 1995: 37-73.
- Oriol R, Danilovs J, Hawkins BR. A new genetic model proposes that the gene is closely related to the gene. Am J Hum Genet. 1981; 33: 421-431.
- Novaretti MCZ. Laboratory Investigation in Patients with Erythrocyte Antibodies. In: Bordin JO, Langhi, DM, Covas DT. Hemotherapy: Fundamentals and Practice. São Paulo: Editora Atheneu. 2007: 186-189.
- Oliveira MBSC, Ribeiro FC, Vizzoni AC. Basic concepts and applied in immuno-hematology. Polytechnic School of Health Joaquim Venâncio. Rio de Janeiro 2013; 50-51.
- Klein HG, Anstee DJ. ABO, H, LE, P1PK, GLOB. Blood groups systems. Mollison's - Blood Transfusion in Clinical Medicine. 12th Hoboken. USA. Publisher: Wiley-Blackwell. 2014: 118.
- Rauws EAJ, Tytgat GNJ. The cure of duodenal ulcer associated with Helicobacter pylori Lancet. 1990; 335: 1233-1235.
- INTITUT VIRION \ SERION. SERION ELISA classic: Helicobacter pylori IgA/IgG/IgM. Accessed on: 26 Nov. 2014.
- Aguiar DCF, Corvelo TCO, Araújo M, Cruz EM, Daibes S, Assumpção MB. Expression of ABH and Lewis antigens in chronic gastritis and pre-neoplastic alterations of the gastric mucosa. Arq Gastroenterol. 2002; 39: 223-231.
- Novaretti MCZ, Dorlhiac-Liacer PE, Chamone DAF. Study of blood groups in Caucasoid and Negroid blood donors in the city of São Paulo. Brazilian Journal of Hematology and Hemotherapy. 2000; 22: 23-29.
- Gaensleen RE, Bell SC, Lee HC. Distributions of genetic markers in the United Sates populations: I blood group and secretor systems. J Forensic Sci. 1987; 32: 1016-1058.
- Barbosa LF. Distribution of ABH and Lewis blood group phenotypes in Helicobacter pylori Completion of course work. Federal University of Pará. Belém. 2011: 15-16.
- Critchley IA, Douglas LJ. Role of glycosides as epithelial cell receptors for Candida albicans. J Gen Microbiol. 1987; 133: 637-643.
- Ura S, Rubio EM, Silva EA. Lewis blood system in patients with leprosy. Hansen.Int. 2000; 25: 115-120.
- Henry S, Oriol R. Samuelsson. Lewis histo-blood group system and associated secretory phenotypes. Vox 1995; 69: 166-182.
- Hynes SO, Moran AP. Comparison of three serological methods for detection of Lewis antigens on the surface of Helicobacter pylori. FEMS Microbiology Letters, Ireland. 2000; 190: 67-72.
- Simoons-Smit IM, et al. Typing of Helicobacter pylori with Monoclonal Antibodies against Lewis Antigens in Lipopolysaccharide. Journal of Clinical Microbiology, Sept. 1996; 34: 2196-2200.
- Young SK, Mei Y, Steven H, Itzkowitz, Qibing S, Tokyo K, et al. Expression of LeY and Extended LeY Blood Group-Related Antigens in Human Malignant, Premalignant, and Nonmalignant colonie Tissues. Cancer Research. 1986; 46: 5985-5992.
- Ravn V, Dabelsteen E. Tissue distribuition of histo-blood group antigens. APMIS. 2000; 108: 1-28.
- Logan RPH, Walker MM. Epidemiology and diagnosis of Helicobacter pylori BMJ. 2001; 323: 920-922.
- Urita Y, Hike K, Torii N, Kikuchi Y, Kurakata H, Kanda E, et al. Comparison of sérum IgA and IgG antibodies for detecting Helicobacter pylori Inter Med. 2004; 43: 548-552.
- Sudraba A, et Al. Performance of Routine Helicobacter pylori Tests in Patients with Atrophic Gastritis. J Gastrointestin Liver Dis December. 2011; 20: 349-354.