
Special Article – Mycology
J Bacteriol Mycol. 2018; 5(5): 1077.
Combining Fluconazole and Leaf Extracts from Vernonia adoensis Enhances the Antifungal Effects on Candida krusei
Nyamuriya R, Sithole S and Mukanganyama S*
Department of Biochemistry, University of Zimbabwe, Mt. Pleasant, Harare, Zimbabwe
*Corresponding author: Stanley Mukanganyama, Biomolecular Interactions Analyses Group, Department of Biochemistry, University of Zimbabwe, Pleasant, Harare, Zimbabwe
Received: August 01, 2018; Accepted: September 07, 2018; Published: September 14, 2018
Abstract
Background: Invasive infections or colonization with Candida krusei have increased due to the prophylactic use of antifungals and widespread use to suppress fungal infections in HIV infected individuals. C. krusei is intrinsically resistant to fluconazole, an azole antifungal which works principally by inhibiting the cytochrome P-450 14a- demethylase and C- 14a- demethylase required for ergosterol synthesis. The aim of the study was to investigate if leaf extracts of Vernonia adoensis can enhance the effect of fluconazole on C. krusei. Eight solvent extracts of the V. adoensis leaves were prepared and these included DCM/Methanol extracts, hexane, DCM, ethyl acetate, ethanol, methanol and water extracts. The extracts were tested for their potency on inhibiting the growth of C. krusei. The MIC were determined from the most potent extract. The effects of combining fluconazole and the most potent extract were also investigated as well as the effect of the drug/plant extract combination on the efflux of rhodamine 6G from C. krusei. The distilled water extract was the most potent in inhibiting growth of C. krusei and was, therefore, combined with fluconazole to determine if there was enhanced effects. The combination lowered the minimum inhibitory concentration of fluconazole on C. krusei from 125 μg/ml to 32 μg/ml. Exposure to fluconazole caused a leakage of 1.03 μg/ ml of cellular proteins but when fluconazole was combined with the distilled water leaf extract, the protein leakage increased to 4.13 μg/ml. The distilled water extract enhanced the inhibition of transported across the membrane shown by increasing R6G concentration of 3.56μM with fluconazole to 4.46μM it was combined with fluconazole. In conclusion, the distilled water leaf extract enhanced the antifungal activity of fluconazole. This action may be due to increased protein leakage and inhibition of transport across the cell membrane. There is need to isolate the active chemical entities from V. adoensis so that they can be used as templates for design of novel antifungals with antifungal potentiation activities.
Keywords: Vernonia adoensis; Candida albicans; Candida krusei; Drug/ Herb Combinations; Candidiasis
Introduction
Candida species are found globally and are part of the human normal flora [1]. Candida infections are found more in people whose immune has been compromised leading to extensions in hospital treatment and death that is significant [2]. The chief source of bloodstream fungal infections are caused by Candida species mainly C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei, which is the fifth in dominancy [3]. Candidemia is generally linked with an increase in morbidity leading to a rise in healthcare costs [4]. In HIV/AIDS patients, Candida species are the main cause of fungal infections. During the early and late phases of an HIV infection, oesophageal candidiasis and oropharyngeal candidiasis can develop due to Candida species colonisation in the oral mucosa [5]. Compared to the HIV negative women, vulvovaginal candidiasis is experienced more in HIV positive women [6]. Candida species can cause systemic and local infections in patients [7].
C. krusei is a fungal pathogen that has a possibility of being multi-drug resistant fungi due to its reduced susceptibility to amphotericin B and flucytosine as well as its intrinsic resistance to fluconazole [8]. However, in vitro, C. krusei remains susceptible to voriconazole as the drug binds more effectively to the C. krusei cytochrome P-450 isoenzyme [9]. Nosocomial infections are a main cause financial problem on patients, morbidity and death. When immunocompromised patients are suffering from a disease or undergo a surgery, they are at risk of nosocomial infections and are more affected if they are admitted in the intensive care unit [10]. The urinary tract, bloodstream, respiratory tract and surgical positions are the major sites of nosocomial infections [11]. The ability of Candida species to form biofilms are a possible cause of nosocomial infections. Biofilms are microbial adherences on abiotic or biotic surfaces to form a polymer matrix, which becomes the microbes’ growth substrate [12]. Invasive fungal infections are infections where fungi get into deep tissues and establish themselves leading to a prolonged illness [12]. In patients who are immunocompromised, the main cause of illness and death are opportunistic invasive fungal infections [13]. The prophylactic use of antifungals has been linked to invasive infections or C. krusei colonisation [2]. Invasive fungal infections are being increased by medical developments leading to a population that is prone to repressed immunological defenses against fungal infections [14].
Presently, over 25% of all artificial medicines have been developed after the discovery of chemical compounds, from plants, that have pharmacological values [15]. Despite advances observed in modern medicine, plants still contribute largely to health care and many modern pharmaceutical drugs contain plant ingredients [16]. The value of plant medicines is due to their phytochemical constituents [17]. Vernonia adoensis belongs to the Asteraceae family. It has a great anti-plasmodia activity [18]. Sexually transmitted diseases such as gonorrhoea are treated using the plant roots of V. adoensis [19]. V. adoensis leaves, contain phenols, flavonoids, terpenoids, steroids, saponins, alkaloids and tannins [20]. V. adoensis, leaves have a lot of pharmacological value in the treatment of sore throat infections, food poisoning and stomach problems [15].
Fluconazole is efficient in treatment of most Candida species except C. krusei [14,20]. Fluconazole is very active against numerous pathogens which results in systemic mycoses. C. krusei is intrinsically resistant to fluconazole [4]. People infected with fluconazole-resistant strains are at a higher risk of reinfection than those infected with susceptible strains [6].
Combination therapy has possible benefits, which comprise less resistant organisms, increased potency of antifungal effectiveness, and reduced toxicities as lesser doses will be administered. The micro dilution checkerboard is one of the traditional methods used for the evaluation of antifungal synergy with synergy determined as the viable growth of an organism that is reduced as a result of a combination of two drugs or a drug and an extract compared with most effective antifungal when tested alone [22]. Synergy requires a four-fold reduction in the MIC of antifungals in combination compared with each use alone [23]. There have been reports that show enhancement of antifungal activity by natural products when combined with conventional antifungals. One such report by Hirasawa and Takada (2004) showed that the catechins from green tea resulted in enhanced antifungal effect of amphotericin against C. albicans [24]. The aim of the study was to investigate if leaf extracts of V. adoensis can enhance the effect of fluconazole on C. krusei.
Materials and Methods
Chemicals used were obtained from Sigma-Aldrich (Steinheim, Germany). The list included Sabouraud dextrose broth and agar, glucose, reserpine, Rhodamine 6G, NaOH, sodium azide, glycine, HCl, sodium phosphate monobasic dehydrate and potassium phosphate dibasic trihydrate, anhydrous sodium carbonate, potassium sodium tartrate, bovine serum albumin and Folin and Ciocalteu’s phenol reagent. Methanol, ethanol, hexane, ethyl acetate, acetone and dichloromethane were obtained from Labchem (Edernvale, South Africa). C. krusei as well as the reference control fungi C. albicans (ATCC 10231) were obtained from the Department of Microbiology, Medical School, University of Zimbabwe, Harare, Zimbabwe. V. adoensis leaves were collected from Centenary (16.8°S, 31.1167°E, and 1156m above sea level), Mashonaland Central Province, Zimbabwe. The plant samples collected (C1E7) were authenticated by Mr Christopher Chapano, a taxonomist at the National Herbarium located at the Harare Botanical Gardens, Harare, Zimbabwe.
Extraction of phytochemicals
Fifty grams of powdered V. adoensis leaves were weighed. In total extraction, 250 ml of dichloromethane (DCM) and 250 ml of methanol were added to a beaker containing the weighed leaves. The mixture was left to stir overnight using a magnetic stirrer. The extract was then filtered [25]. The filtrate was left to evaporate in a fume hood and a fan was used to speed up the evaporation. When the filtrate had dried, the total extract was weighed, put into a falcon tube then stored at 4°C. The residue was discarded. Serial exhaustive extraction was performed using non-polar solvents first to polar solvents. Fifty grams of powdered V. adoensis leaves were weighed. The first solvent that was used for extraction was 400 ml of hexane. Hexane was added to 50 g of powdered V. adoensis leaves, which were in a beaker, and the mixture was left to stir overnight on a magnetic stirrer. The extract was then filtered from the marc and the filtrate was concentrated by evaporation in the fume hood. The residue was dried and 400 ml of DCM was added to it and left stirring overnight then filtered. After DCM, 400 ml of acetone, ethyl acetate, ethanol, methanol and distilled water were added, each time drying the residue after extraction. The filtered extracts were dried in a fume hood at ambient temperature (25oC) using a fan and the dried extracts were weighed, put in labelled falcon tubes and stored at 4°C.
Cell culturing
The media was prepared from Sabouraud dextrose broth (SDB). Thirty grams of SDB was dissolved in 1000ml of distilled water and autoclaved. Two centrifuge tubes were used and these were labelled control and C. krusei isolate 1 and C. krusei isolate 2. A volume of 20ml of SDB was added to each tube. In the growth tube, 20μl of inoculum from glycerol stock were added and in the control tube, there were no additions [25]. The tubes were incubated at 37oC overnight in an incubator shaking at 120 revolutions/minute. To culture in agar plates, 47g of Sabouraud and 2% glucose agar were dissolved in 1000 ml of boiled distilled water then autoclaved. The agar was poured into petri dishes and allowed to solidify. A volume of 20 μl of inoculum was pipetted at the centre and spread using a glass spreader that has been sterilised in 70% and burnt over the flame to remove the ethanol. The plates were left to incubate at 37°C overnight in an incubator without shaking.
Determination of minimum inhibitory concentrations (MIC)
The assay to determine the least concentration of the extracts and of the antifungal drug fluconazole that would inhibit the growth of C. krusei (both isolate 1 and isolate 2) was carried out according to CLSI reference method [26]. After incubating for 16-24 hrs at 37°C cells were diluted in relation to the McFarland’s standard [27]. The solution of 0.5 McFarland’s must be in a range of 0.08 to 0.10 optical density at 600nm and would contain 1.5x108 CFU/ml of cells. Calculations were done so that the final cell concentrations in the micro titre well was 2×106 CFU/ml of cells.
Cells were exposed to extracts or fluconazole in a 96-well plate. The concentration of the standard antibiotic ranged from 0-1 μg/ml. When assessing the effects of the extracts, the drug was substituted with extracts at concentrations ranging from 100 μg/ml to 0 μg/ml. The plates were sealed with parafilm™ paper and incubated in a container containing wet paper towel over night at 37°C in a non-shaking incubator (Jeio Tech, Korea). The MIC was defined by comparing the amount of growth in the test tubes and the growth control tubes as reported by Santos and Hamdan (2005) and recommended by the Clinical and Laboratory Standards Institute [28].
Determination of the combination of fluconazole and extract on growth of C. krusei
The assay was carried out following the procedure by Ahmad et al., [29] to evaluate if there was enhanced antifungal activity when fluconazole and the leaf extract were combined The final cell concentrations in the 96-well microtitre wells was 2×106 CFU/ml of cells. To each well, 50 μl of the drug was added to 50 μl of the extract then 100μl of the diluted cells were added. The plate was left in a nonshaking incubator (Jeio Tech, Korea) at 37oC.
Protein leakage assay
Determination of protein leakage was carried out according to the method by Ladokhin et al., [30], C. krusei was sub-cultured in 20 μl of Sabouraud Dextrose broth media in a falcon tube and left to incubate overnight at 37°C with shaking at 120 rpm. A volume of 200 μl of the sub-cultured cells were added to 200 ml of SDB media in a 1 litre container and left to incubate overnight at 37°C with shaking at 120 rpm. The cells were then poured into 4 centrifuge tubes, distributing them equally. The tubes were then centrifuged at 3000 rpm for 5 min and the supernatant was discarded. The pellets were diluted with 0.9% saline until their cell density was 1.526 at 600 nm. To 5 centrifuge tubes which were labelled A, B, C, D and E, 6mls of cells were added. To A, a volume of 120 μl of 5 mg/ml stock of the distilled extract and 96 μl of 2 mg/ml fluconazole were added. The combination concentrations were 100μg/ml extract and 32 μg/ ml fluconazole. To B, only 96 μl of 2 mg/ml fluconazole was added. To C, only 120 μl of 5 mg/ml stock of the distilled extract was added. To D, 216 μl of sterile distilled water was added and to E, 600 μl of 1% SDS so that the final concentration was 0.1%. The tubes were left to incubate for 2 hr at 37°C in a shaking incubator SI-300, incubator Lab companion, (Jeio Teicho, Korea) and then centrifuged at 3500 rpm for 4 min. The pellet was discarded the supernatants were used for the protein determination assays. The controls were D (cells only) and E (0.1% SDS). Protein content was measured by the method of Lowry (1951), using BSA as a standard.
The effects of extract/fluconazole combination on drug efflux in C. krusei
The determination of drug efflux assay was carried out according to the method by Maesaki et al [31]. Using rhodamine 6G as a probe substrate for the efflux pumps. C. krusei was first cultured in a sterile 50 ml centrifuge tube and then 200 μL of the cells were inoculated into 2000 μL of Sabouraud dextrose broth and left in an incubator overnight at 37°C shaking at 120 rpm. The cells were divided into 6x50 ml centrifuge tubes and centrifuged at 4000rpm for 10ml using Hettich Rotofix 32 Centrifuge, (Tuttingen, Germany). The supernatant was discarded and the cells were washed twice in phosphate buffered saline (PBS) pH 7.2 by mixing the cells with the buffer, centrifuging at 4000 rpm for 10 min and discarding the supernatant. The pellet was collected in a weighed tube and the mass of the pellet was weighed before being suspended in PBS containing 10mM NaN3 at a density of 40mg/ml. Rhodamine 6G (R6G) was immediately added to the cells to achieve a final concentration of 10 μM. The cells were incubated at 120 rpm for 1 hour. The cells were divided into two tubes A and B in the ratio 1:2. The tubes were centrifuged at 4000 rpm for 5 min. After discarding the supernatant, the pellet in tube A was resuspended in PBS at 40 mg/ml while the pellet in tube B was resuspended in PBS containing 1M glucose at 40 mg/ml. Tube B was further divided into 10 tubes each with 5 ml of the cells suspended in 1M glucose. To tubes B1 and B2, 60 μg/ml of reserpine was added. To tubes B3 and B4, fluconazole was added such that the final concentration of fluconazole was 32 μg/ml. To tubes B5 and B6, the distilled water leaf extract of V. adoensis was added such that the final concentration of the extract was 100 μg/ml. To tubes B7 and B8, a combination of fluconazole and the distilled water extract was added, the same volumes added in B3 and B5. Tubes B9 and B10 were controls with glucose only. The contents of the tubes were mixed and incubated in an incubator at 37°C shaking at 120 rpm for 30 minutes. The tubes were then centrifuged at 4000 rpm for 10min and the supernatants were collected in other clean tubes. The pellets were resuspended in 5 ml of 0.1M glycine HCl, pH 3 to lyse. The cells were incubated for 18 hours at 37°C shaking at 120rpm. After, the tubes were centrifuged at 4000 rpm for 10 minutes and the supernatant was collected. The absorbance of R6G was measured at 527 nm using the Unico 2100 UV spectrophotometer, (United Product and Instruments Inc, USA). The standards were prepared by diluting 10 μM of R6G in glycine HCl.
Reading of MICs and Statistical analyses
The absorbance of the plate was read at 590nm before and after incubation using a Multimode micro plate reader, GENios Pro, (TECAN Austria G.M.B.H, Untersbergstrasse, Austria). The difference in the absorbance values between pre-incubation and post incubation was used to plot a graph using the GraphPad prism 5 for Windows (GraphPad Software Inc., San Diego, California, USA version 5.03). Statistically significant differences between the mean of the controls and the tests were analysed using one-way ANOVA with Dunnett’s multiple comparison post-test. Enzyme kinetics was analysed with nonlinear regression and allosteric sigmoidal using GraphPad Prism 5 (Version 5.03 GraphPad Software Inc. San Diego, California U.S.A).
Results
Extraction of phytochemicals from Vernonia adoensis leaves
The V. adoensis DCM/Methanol (1:1) had the highest yield (10%) followed by distilled water extract (5%), methanol (4%) and acetone (2%) as shown in Table 1. The yields did not depend on the polarity of the solvent used for extraction.
Plant extract
Masses (g)
% yield
DCM/Methanol (1.1)
5.047
10.1
Hexane
0.53
1.1
DCM
0.345
0.69
Acetone
1.15
2.3
Ethyl acetate
0.211
0.422
Ethanol
0.431
0.862
Methanol
2.053
4.11
Distilled water
2.519
5.04
The total extract had the highest yield and the ethyl acetate extract had the least yield. Polarity of the solvent used for extraction did not determine the quantity of the yield.
Table 1: The masses of different solvent extracts after they were dried.
Determination of MIC of fluconazole on C. albicans and C. krusei
With increase in concentration of fluconazole, the cell density decreased. Comparing all the columns with media, the MIC for C. albicans was found to be 8 μg/ml (Figure 1A). The MIC of fluconazole on C. krusei was 125 μg/ml (Figure 1B) which is higher than that of C. albicans. The MFC for fluconazole on C. krusei was also 125 μg/ ml. Thus, C. krusei, which is intrinsically resistant to fluconazole, was found to be less sensitive to the effects of fluconazole when compared to C. albicans. Subsequent work was then conducted on C. krusei only.
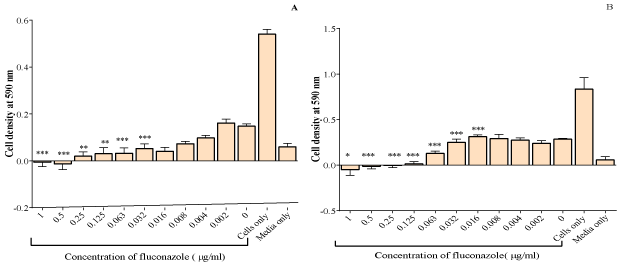
Figure 1: The effect of fluconazole on C. albicans (A) and C. krusei (B). The MIC for C. albicans was 8μg/ml and that of C. krusei was 125μg/ml. The values plotted
are the mean ± standard deviation at n=5. The test of significance was carried out by comparing all samples to media only *p< 0.05, **p<0.01 and ***p<0.001.
The effects of the V. adoensis extracts on C. krusei
The total extract DCM, acetone, ethyl acetate, ethanol and methanol extracts had no effect on inhibiting the growth of C. krusei, as the cell densities were high. Significant potency was generally shown for the highest concentrations although there was no MIC. Only the distilled water extract significantly reduced the growth of the fungi (Figure 2). The effects of all the V. adoensis extracts at concentration 100 μg/ml were plotted together for comparison and cell viabilities of each extract were calculated as a percentage as shown in Figure 3. The methanol extract had the least effect on inhibiting the growth of C. krusei and the distilled water was the most potent extract with a cell viability of only 13.1% at 100 μg/ml of extract concentration.
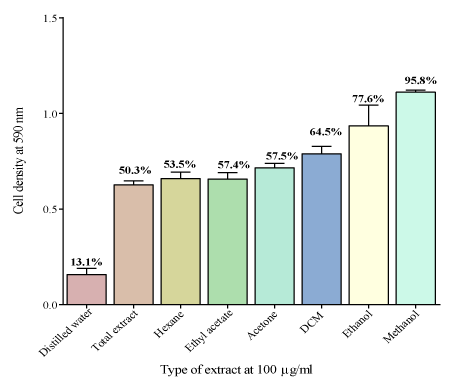
Figure 2: The effect of different solvent leaf extracts at 100 μg/ml on the
growth of C. krusei. Distilled water extract was the most potent extract with a
cell viability of 13.1% and the methanol extract was the least potent with a cell
viability of 95.8%. The potency of the extract did not depend on the polarity
of the solvent. The alcohol extracts of V. adoensis had the least effect growth
inhibition of C. krusei.
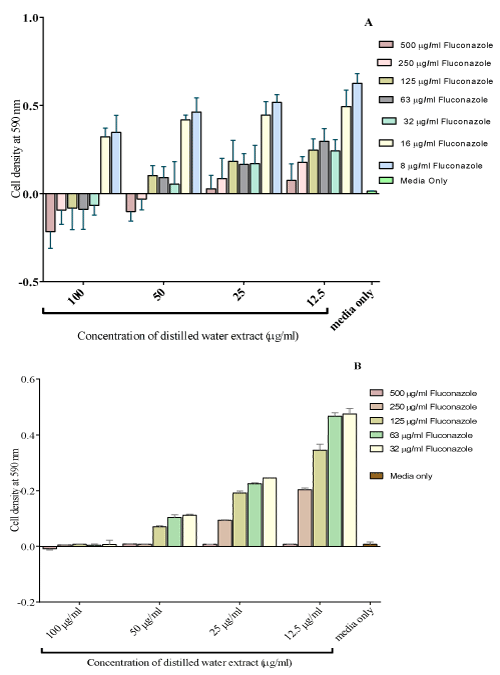
Figure 3: The effect combining fluconazole with the distilled water extract on
the growth of C. krusei (Isolate 1-A and Isolate 2-B). The MIC of fluconazole
was reduced to 32 μg/ml combined when combined with 100 μg/ml of distilled
water extract from the original value of 125μg/ml of for fluconazole only.
The effect of combining the most potent extract and fluconazole on C. krusei
Concentrations of fluconazole ranging from 500 μg/ml to 8μg/ ml were combined with the distilled water extract from 100 μg/ml to 12.5 μg/ml. An MIC of the combination of 100 μg/ml of the distilled water extract and 32 μg/ml of fluconazole was obtained (Figure 4). As the distilled water extracts concentrations increased, the cell densities decreased with a greater decrease observed at higher concentrations of fluconazole. Combining different concentrations of fluconazole with 100 μg/ml of the distilled water extract increased the potency of fluconazole. The MIC was at a combination of 32 μg/ml of fluconazole with 100 μg/ml of the distilled water extract.
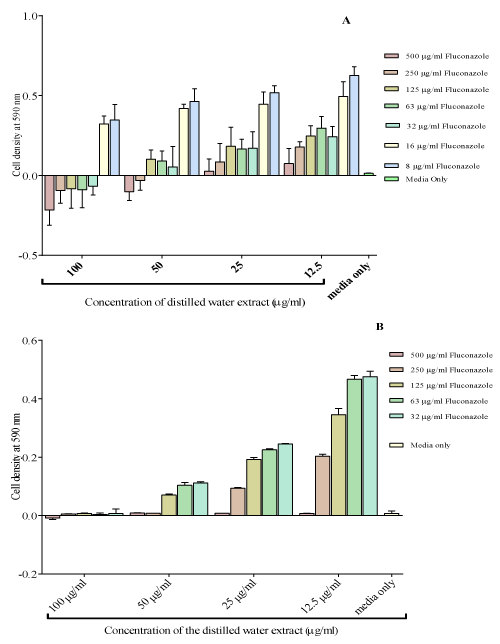
Figure 4: The effect combining the distilled water extract (Isolate 1-A and
Isolate 2-B). The distilled water was the most potent extract. The MIC of
fluconazole was reduced to 32μg/ml combined with 100 μg/ml of distilled
water extract from 125μg/ml of fluconazole only.
Effect of the plant extract/drug combination on protein leakage
Having noted the lowest combination of fluconazole and the extract that reduced the growth C. krusei, we decided to explore a possible mechanism of action of this combination termed potent combination. C. krusei cells were separately exposed to SDS, fluconazole and the potent combination. The protein concentrations of supernatants determined. In the control supernatants, there was some basal protein leakage. There was more protein leakage to cells that were exposed to the distilled water extract as well as in the cells that were exposed to the combination of the drug and plant extract compared to cells only (Figure 5). Cells exposed to SDS had the highest protein leakage concentration.
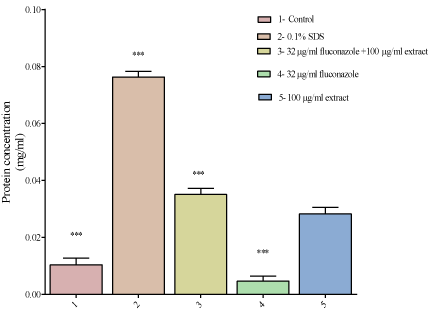
Figure 5: The effect of exposing C. krusei to different concentrations of
fluconazole, the extract and a combination of the drug and extract on protein
leakage. There was more protein leakage in the cells exposed to SDS of
0.076 mg/ml then in the combination which had a protein concentration
of 0.037 mg/ml. Cells exposed to the distilled water extract only had a
protein concentration of 0.035 mg/ml. The control (cells only) had a protein
concentration of 0.01mg/ml and the cells that had been exposed to fluconazole
had a protein concentration of 0.0046mg/ml. There was more protein leakage
in C. krusei cells exposed to SDS and the least protein leakage was in C.
krusei cells exposed to fluconazole.
The effect of the plant extract/drug combination on drug efflux and accumulation
The concentrations of R6G that accumulated in C. krusei exposed to PBS, reserpine, fluconazole, V. adoensis, distilled water leaf extract and glucose are shown in Figure 6A. On assessing the efflux, there was more R6G efflux of R6G in the C. krusei cells that were exposed to fluconazole than in cells which were treated with the V. adoensis distilled water leaf extract only. The least efflux of R6G was in cells, which had PBS only, and most efflux of R6G was in cells, which had glucose only. On accumulation, the C. krusei cells, which were exposed to PBS, had the most accumulation of the dye. There was more accumulation of R6G in cells treated with the V. adoensis distilled water leaf extract compared to the cells which were exposed to fluconazole (Figure 6B). Combining the leaf extract and fluconazole resulted in more accumulation of R6G showing that the extract inhibited the efflux pump causing accumulation of R6G.
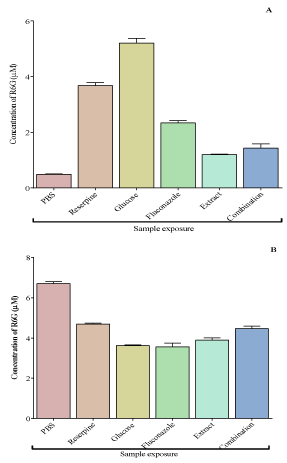
Figure 6: The efflux (A) and accumulation (B) of R6G in cells exposed to
PBS, reserpine, glucose fluconazole and V. adoensis distilled water leaf
extract. There was more efflux of R6G in C. krusei cells that was exposed
to glucose only and the least efflux was in cells that had PBS only. In
cells treated with fluconazole, there was more efflux of R6G than in cells
treated with the extract only. Comparing with the efflux in cells treated with
fluconazole, combining fluconazole and the leaf extract reduced the efflux.
There was more accumulation of R6G in cells treated with the V. adoensis
distilled water leaf extract only compared to the cells which were treated with
fluconazole only. Combining the leaf extract and fluconazole resulted in
more accumulation of R6G showing that the extract inhibited the efflux pump
causing accumulation of R6G. There was more accumulation of R6G in cells
treated with reserpine than in cells which had glucose only.
Discussion
Presently, over 25% of all artificial medicines have been developed after the discovery of chemical compounds, from plants, that have pharmacological values [15]. Despite advances observed in modern medicine, plants still contribute largely to health care and many modern pharmaceutical drugs contain plant ingredients [16]. Fluconazole is efficient in treatment of most Candida species except C. krusei [14]. Plants may contain compounds that do not have essential antimicrobial activity but are able to make a pathogen sensitive to the antimicrobial drug that was ineffective. Combination therapy can be used to develop the antimicrobial spectrum to obtain synergistic antimicrobial activity, to prevent the emergence of resistant mutants and to minimise toxicity [32]. This study was conducted to determine the effect of combining fluconazole and V. adoensis leaf extracts on Candida krusei, fungi that is intrinsically resistant to fluconazole.
The MIC of fluconazole on C. albicans found in this study was 16 μg/ml and that of C. krusei was 125 μg/ml supporting data in literature that C. krusei is intrinsically resistant to fluconazole [4]. Four possible mechanisms of resistance are decreased drug concentration, upregulation of the target enzyme, target site alteration and development of bypass pathways [33]. Reduced accumulation of fluconazole is not the main mechanism of resistance in C. krusei but the main mechanism of resistance is reduced susceptibility of 14a-demethylase [34]. C. krusei is intrinsically resistant meaning establishment of resistance occurred naturally without pre-exposure to the drug, hence, efflux pumps are not a possible mechanism of resistance [33]. Fluconazole inhibits 14 a- lanosterol demethylase in ergosterol biosynthetic pathway leading to the accumulation of lanosterol and toxic 14a-methylated sterols in the fungicidal membrane. The accumulation causes the cell membrane to be defective, decreases the accessibility of ergosterol and alters the permeability of the fungal cells [35].
At 100 μg/ml of each extract, the distilled water had the highest inhibitory effect on the growth of C. krusei with a cell viability of 13.1% and methanol had the least growth inhibitory effect with a cell viability of 95.8%. Since polar solvents were used to extract both extracts, the phytochemicals were expected to be the same or have a similar effect but the alcohol extracts of V. adoensis leaves had the least effect on inhibiting the growth of C. krusei. The effect of the extracted plant phytochemical depends on particle size, degree of processing, origin of the extract, moisture content and the nature of the plant material [35]. V. adoensis, leaves have been reported to contain phenols, flavonoids, terpenoids, steroids, saponins, alkaloids and tannins [15]. Water extracts contain tannins that inhibit fungal growth, which might have been the reason why the distilled water extract was the most potent. Flavonoids complex with the cell wall and binds to adhesins whilst the polyphenols and tannins bind to adhesins, inhibits enzymes, deprives substrates and complex with the cell wall causing membrane disruption and metal ion complexion [35]. Terpenoids and essential oils also cause membrane disruption in fungi. The alkaloids intercalate into the cell wall and DNA of parasites and inhibits the release of autacoids and prostaglandins [25]. All the phytochemicals mention above are extracted with water making the distilled water extract have the most inhibitory activity [20].
The MIC of fluconazole alone was 125 μg/ml and whilst there was no MIC for the distilled water extract. However, when the two were combined, the MIC of fluconazole decreased to 32 μg/ml. Increasing the extract concentration caused a decrease in the MIC of fluconazole; hence, the distilled water extract had a synergistic effect on the growth inhibitory of C. krusei. The proposed mechanism of resistance of fluconazole are decreased drug concentration, upregulation of the target enzyme, target site alteration or development of bypass pathways [33]. Similar to the action of fluconazole, the distilled water extract in this study might have targeted the ergosterol biosynthesis pathway, increasing the fluidity for the depletion of the ergosterol [36]. The extract might have had some membrane activity either in enhancing the membrane damage by interfering with ergosterol synthesis in the cell membrane of C. krusei or facilitating the uptake of the fluconazole, hence, increasing the inhibition of 14a-sterol demethylase which converts lanosterol to ergosterol [37]. Thus, the combined effect of fluconazole and the V. adoensis distilled water extract might have been related to the inhibition of membrane sterol synthesis. The extract might also have converted the action of fluconazole from fungistatic to fungicidal [38]. Diminished sensitivity of the target enzyme cytochrome P450 sterol 14a-demethylase (CYP 51) to inhibition by azole agents is the main mechanism of fluconazole resistance. The diminished sensitivity might be due to the decreased drug concentration, as efflux pumps would pump some of the drug out. Combining fluconazole with the distilled water extract could have caused more of the drug to reach the target site as the efflux pumps were inhibited by the extract causing the drug to remain inside the C. krusei [39].
To determine the action of the extract, the effects on membrane integrity were done by exposing C. krusei to the extract. C. krusei cells, which were exposed to 0.1% sodium dodecyl sulphate (SDS), had the highest concentration of protein of 0.076 mg/ml. SDS is an anionic detergent or surfactant, which is a strong protein denaturant. SDS interacts with the protein in a hydrophobic manner [30]. The expansion of the protein chain at micellar concentration of SDS is driven by columbic repulsion between the protein bound micelles and the anionic amino acid side chains. Membrane proteins have a greater hydrophobic content so interacts more with the SDS, which linearize the proteins and impart a negative charge to linearized proteins, as it is an anionic detergent. There was high protein leakage in the cells exposed to SDS because it linearized the membrane proteins causing a higher protein leakage [40]. The cells, which were exposed to the combination of the drug and the distilled water extract, had a protein concentration of 0.037 mg/ml, which was higher compared to those of cells that were not exposed to anything that was 0.01 mg/ ml. The extract might have had some membrane disrupting activity that facilitated the uptake of fluconazole or might have interacted directly with the ergosterol biosynthesis pathway causing plasma membrane damage or cell shrinkage causing it to be more permeable [41]. Fluconazole had no effect in causing protein leakage because the C. krusei cells are intrinsically resistant to fluconazole and 32μg/ ml was a very low concentration to have an effect on membrane permeability [39]. Permeabilization of membranes is a vital feature in the interactions of proteins and other molecules with lipid bilayers. One of the most effective killing mechanisms of peptides with antimicrobial, cytotoxic or host defence functions is induced leakage of a cell’s contents. Leakage of molecules spontaneously from lipid vesicles is vital for drug encapsulation and delivery [30].
The effects of the extracts were investigated on transport of molecules across cells of C. krusei. Efflux pumps are transport proteins involved in the extrusion of toxic substances including virtually all classes of clinically relevant antibiotics from within cells into the external environment [42]. Rhodamine 6G was used as the probe substrate for the ATP-dependent transporters in C. krusei. There was more concentration of rhodamine 6G in the cells treated with reserpine the standard efflux inhibitor than in cells with glucose only because glucose is an energy supplier that increases the activity of efflux pumps. All ATP-dependent drug efflux proteins are members of the ABC superfamily. The ABC transporters act as molecular pumps that actively translocate drugs through the plasma membrane by using energy gained from ATP hydrolysis [43]. The transporters are sensitive to reserpine, an ATP-dependent efflux pump inhibitor. Reserpine is extensively bound to plasma proteins. The mechanism of action of reserpine is through inhibition of the ATP/Mg2+ pump [42]. Cells exposed to phosphate buffered saline served as the negative control and since there was no glucose to provide the energy, there was more accumulation of R6G compared to all the other samples. There was more accumulation of R6G in cells exposed with the V. adoensis distilled water leaf extract compared to cells treated with fluconazole only. The combination of the drug and the extract resulted in even more accumulation of the R6G. The extract inhibited the efflux pumps. Efflux pump inhibitors block the pumps restoring drug activity in resistant cells [44]. The distilled water extract might have helped in restoring the activity of fluconazole by increasing its accumulation resulting in enhanced antifungal activity. An efflux pump inhibitor will bind to the pump with an increased affinity, competitive inhibition, or impair access to the binding site for the drug, non-competitive inhibition [31].
Conclusion
The leaf extract of V. adoensis was found to enhance the antifungal effect of fluconazole on C. krusei in vitro. Fluconazole alone did not cause any protein leakage from C. krusei but when the drug was combined with the distilled water leaf extract of V. adoensis, there was increased protein leakage. The distilled water leaf extract of V. adoensis increased the accumulation of R6G and the effects where enhanced when combined with fluconazole. Phytochemicals from V. adoensis could provide leads to chemical entities that might restore the sensitivity of C. krusei to fluconazole. There is need to isolate and purify the phytochemicals from V. adoensis so as to evaluate their effectiveness when combined with fluconazole.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Acknowledgement
Support from the International Science Programmes (ISP) through the International Program in the Chemical Sciences (ISP IPICS: ZIM01, Uppsala University, Uppsala, Sweden) and the International Foundation in Sciences (IFS F/3413-03F, Stockholm, Sweden) us acknowledged. F/3413-03F supported research under the title: “Screening natural plant products from selected plants from Zimbabwe as a source of anti-infective compounds for phytomedicines development”. ISP IPICS: ZIM01 supported the research under the title “Biomolecular Interactions Analyses”. The authors acknowledge the assistance of Mr. Christopher Chapano, a taxonomist with the National Herbarium and Botanical Gardens, Harare, Zimbabwe in the authentication of the plant sample names.
References
- Paramythiotou E, Frantzeskaki F, Flevari A, Armaganidis A and Dimopoulos G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules. 2014; 19: 1085-1119.
- Hautala T, Ikaheimo I, Husu H, Saily M, Siitonen T, Koistinen P, et al. A cluster of Candida krusei infections in a haematological unit. Bio Med Central Infectious Diseases. 2007; 7: 97.
- Plantinga TS, Johnson MD, Scott WK, Oosten L, Van der Meer JWM, Perfect JR, et al. Human genetic susceptibility to Candida infections. Medical Mycology. 2012; 50: 785-794.
- Nascimento MDSB, Leitao VMS, Neto si lva MAC, Maciel LB, Filho Muniz WE, Viana GMC, et al. Eco-epidemiologic study of emerging fungi related to the work of babaçu coconut breakers in the State of Maranhão, Brazil. Rev Soc Bras Med Trop. 2014; 47: 74-78.
- Perovic O and Singh A. Nosocomial infections in Patients with Human Immunodefiency Virus. InTech Journals. 2011; 1: 151-164.
- Zhang L, She X, Merenstein D, Wang C, Hamilton P, Blackmon A, et al. Fluconazole resistance patterns in Candida species that colonize women with HIV. Current Therapeutic Research. 2014; 76: 84-89.
- Pfaller MA. Nosocomial candidiasis: Emerging species, reservoirs and modes of transmission. Clinical Infectious Diseases. 1996; 22: S89-94.
- Samaranayake YP and Samaranayake LP. Candida krusei: Biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. Journal of Medical Microbiology. 1994; 41: 295-310.
- Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, et al. Candida krusei a multidrug-resistant opportunistic fungal pathogen: Geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program 2001 to 2005. Journal of Clinical Microbiology. 2008; 46: 515-521.
- Enoch DA, Ludlam HA and Brown NM. Invasive fungal infections: A review of epidemiology and management options. Journal of Medical Microbiology. 2006; 55: 809-818.
- Mohammed M, Mohammed AH, Mirza MA and Ghori A. Nosocomial Infections: An overview. International Research Journal of Pharmacy. 2014; 5: 7-12.
- Ramana KV, Kandi S, Bharatkumar V, Sharada CV, Rao R, Mani R and Rao SD. Invasive fungal infections: A Comprehensive review. American Journal of Infectious Diseases and Microbiology. 2013; 1: 64-69.
- Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic Invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clinical Infectious Diseases. 2002; 34: 7-14.
- Zarrin, M and Mahmoudabadi A. Invasive candidiasis. Jundishapur Journal of Microbiology. 2009; 2: 1-6.
- Mutuku NC, Swamy TA and Jackie O. In vitro control of selected pathogenic microorganisms by Vernonia adoensis leaves. International Journal of Bioassays. 2013; 2: 1113-1117.
- Buli GA, Duga AG and Dessalegn E. Antimicrobial activity of Lippia adoensis koseret against human pathogenic bacteria and fungi. American Journal of Clinical and Experimental Medicine. 2015; 3: 118-123.
- Danlami U, David BM, Joyce O, Olutayo O and Thomas SA. The antioxidant potentials and phytochemical properties of the hexane, ethyl acetate and ethanolic extracts of Securinega virosa (Euphorbiaceae) leaves. Journal of Applied Pharmaceutical Science. 2013; 3: 131-133.
- Kisangau DP, Hosea KM, Joseph CC and Lyaruu HVM. In vitro antimicrobial assay of plants used in traditional medicine in Bukoba rural district, Tanzania. African Journal Traditional Complementary and Alternative Medicines. 2007; 4: 510-523.
- Swamy TA, Obey J and Mutuku NC. Phytochemical analysis of Vernonia adoensis leaves and roots used as a traditional medicinal plant in Kenya. International Journal of Pharmacy and Biological Sciences. 2013; 3: 46-52.
- Charlier C, Hart E, Lefort A, Ribaud P, Dromer F, Denning DW and Lortholary O. Fluconazole for the management of invasive candididasis: Where do we stand after 15 years? J Antimicrob Chemother. 2006; 57: 384-410.
- Jain S, Vishawanatha T, Reena V, Divyashree B, Sampath A, Siddhalingeshwara K, et al. Antibiotic synergy test: Checkerboard method on multidrug resistant Pseudomonas aeruginosa. International Research Journal of Pharmacy. 2011; 2: 196-198.
- Rand K, Houck HJ, Brown P and Bennett D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrobial Agents and Chemotherapy. 1993; 37: 613-615.
- Adwan G, Abu-Shanab A and Adwan K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug- resistant Pseudomonas aeruginosa strains. Asian Pacific Journal of Tropical Medicine. 2010; 3: 266-269
- Hirasawa M and Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. Journal of Antimicrobial Chemotherapy. 2004; 53: 225-229.
- Mokgotho MP, Gololo SS, Masoko P, Mdee LK, Mbazima V, Shai LJ, et al. Isolation and chemical structural characterisation of a compound with antioxidant activity from the roots of Senna italica. Evidence-Based Complementary and Alternative Medicine. 2013.
- Wayne PA, Clinical and Laboratory Standards Institute (2008b) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved standard, 3rd edition, M27-A3.
- McFarland J. Nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. Journal of the American Medical Association. 1907; 14: 1176-1178.
- Santos DA and Hamdan JS. Evaluation of Broth Microdilution Antifungal Susceptibility Testing Conditions for Trichophyton rubrum. Journal of Clinical Microbiology. 2005; 43: 1917-1920.
- Ahmad A, Khan A, Khan LA and Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. Journal of Medical Microbiology. 2010; 59: 1178-1184.
- Ladokhin AS, Wimley WC and White SH. Leakage of membrane vesicle contents: Determination of mechanism using fluorescence requenching. Biophysical Journal. 1995; 69: 1964-1971.
- Maesaki S, Marichal P, Bossche HV, Sangland D and Johno S. (1999). Rhodamine 6G efflux for the detection of CDR1- overexpressing azole resistant albicans strains. J Antimicrob Chemother. 1999; 44: 27-31.
- Orozco AS, Higginbotham LM, Hitchcock CA, Parkinson T, Falconer D, Ibrahim AS, et al. Mechanism of fluconazole resistance in Candida krusei. Antimicrobial Agents and Chemotherapy. 1998; 42: 2645-2649.
- Aiyegorom OA and Okoh AI. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. Journal of Medical Plants Research. 2009; 3: 1147-1152.
- Tiwari P, Kumar B, Kaur M, Kaur G and Kaur H. Phytochemical screening and extraction. International Pharmaceutical Sciencia. 2011; 1: 98-106.
- Moosa MS, Sobel JD, Elhalis H, Du W and Akins RA. Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrobial Agents and Chemotherapy. 2004; 48: 161-167.
- Kanafani ZA and Perfect JR. Resistance to antifungal agents: Mechanism and clinical impact. Clinical Infectious Diseases. 2008; 46: 120-128.
- Lepesheva GL and Waterman MR. Sterol 14α-Demethylase Cytochrome P450 (CYP51), a P450 in all Biological Kingdoms. Biochim Biophys Acta. 2007; 1770: 467-477.
- Fiori A and Dijck PV. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrobial Agents and Chemotherapy. 2012; 56: 3785-3796.
- Guinea J, Sanchez-Somolinos M, Cuevas O, Pelaez T and Bouza E. Fluconazole resistance mechanisms in Candida krusei: The contribution of efflux-pumps. Medical Mycology. 2006; 44: 575-578.
- Bhuyan AK. On the mechanism of SDS-induced protein determination. Biopolymers. 2010; 93: 186-199.
- Li H, Zhang C, Chen Z, Shi W and Sun S. A promising approach to overcoming the intrinsic resistance of Candida krusei to fluconazole-combining tacrolimus with fluconazole. Federation of European Microbiological Societies Yeast Research. 2014; 14: 808-811.
- Webber MA and Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003; 51: 9-11.
- Martinez L and Falson P. Multidrug resistant ATP-binding cassette membrane transporters as targets for improving oropharyngeal candidiasis treatment. Advances in Cellular and Molecular Otolaryngology. 2014; 2: 1-8.
- Ohene-Agyei T, Mowla R, Rahman T and Venter H. Phytochemicals increase the antibacterial activity by acting on a drug efflux pump. Microbiology Open. 2014; 3: 885-896.