
Research Article
J Bacteriol Mycol. 2019; 6(2): 1099.
Osteoarticular Infections: Blood as a Determinant Factor in the Isolation of Kingella kingae
Noguera-Julián A1-4, Monsonis M5, Ludwig G5, Moreno-Romo D6 and Gené A5*
¹Infectious Malalties and Inflammatory Systemic Response in Pediatrics, Infection Unit, Pediatric Research Institute Hospital Sant Joan de Déu, Spain
²Department of Pediatrics, University of Barcelona, Spain
³CIBER of Epidemiology and Public Health, CIBERESP, Spain
4Red of Translational Research in Pediatric Infectology, RITIP, Spain
5Microbiology Service, Sant Joan de Déu Hospital, Spain
6Department Orthopedic Surgery and Traumatology, Sant Joan de Déu Hospital, Spain
*Corresponding author: Amadeu Gené, Microbiology Service, San Juan de Dios Hospital, Passeig Sant Joan de Déu, Esplugues de Llobregat, Barcelona, Spain
Received: February 19, 2019; Accepted: March 22, 2019; Published: March 29, 2019
Introduction
We assessed the capacity of Kingella kingae to grow in blood culture bottles, taking into account the concentrations of the microorganism and of blood in the culture medium. An initial suspension (dilution A) of 32 strains of Kingella kingae was serially diluted (dilutions B to F). One mL of all dilutions was inoculated in two blood culture bottles, together with 1mL of human blood in the 2nd bottle. One mL serial dilutions of human blood were added to blood culture bottles previously inoculated with 1 ml of Kingella kingae dilution 1/104 (dilution C). In non-blood-supplemented blood culture bottles, 23 strains grew with dilution A and only one with dilution B, as compared to all strains with dilutions A to D, 22 with dilution E, and one with dilution F in blood-supplemented bottles. In blood culture bottles inoculated with Kingella kingae dilution C and decreasing concentrations of human blood, all strains grew with blood dilutions 1/2 and 1/4, 26 with dilution 1/8, 19 with dilution 1/16, 10 with dilution 1/32, and none with dilution 1/64. Increasing time to positivity was observed with both decreasing bacterial (p=0.001) and blood concentrations (r=-0.632, p<0.0001). The addition of human blood was essential to boost the growth of Kingella kingae in blood culture bottles and may prove useful to improve the isolation of fastidious Kingella kingae organisms from pediatric osteoarticular exudates.
Keywords: Children; Culture; Kingella kingae; Osteoarticular infection
Introduction
Since the early 90’s Kingella kingae has emerged as one of the main causes of osteomyelitis and septic arthritis in children younger than 4 years of age [1-4]. Conventional cultures rarely recover K. kingae from osteoarticular specimens and only the inoculation of Blood Culture Bottles (BCB) allows its isolation in some cases [2,5,6]. However, even when the BCB method is employed, many pediatric skeletal system infections remain culture-negative and only the use of sensitive species-specific molecular detection assays establishes the bacteriological diagnosis of the disease [4,7-10].
The present study analyzes the capacity of K. kingae to grow in BCB, taking into account both the inoculum and the presence and concentration of blood as a growth supplement in the culture medium.
Materials and Methods
Thirty-two K. kingae strains isolated from 32 patients (15 females) aged from 7 to 28 months at diagnosis of osteoarticular infections (24 arthritis and 8 osteomyelitis cases) in Hospital Sant Joan de Déu (Barcelona, Spain) between 1997 and 2015 were studied.
From each of the strains, a microorganism suspension with a theoretical bacterial concentration of 1.5×108 Colony Forming Units (CFU)/mL (McFarland 0.5) was prepared (dilution A). Dilution A was then serially diluted 1/103, 1/104, 1/105, 1/106, and 107 (dilutions B, C, D, E, and F, respectively) by addition of sterile 0.9% w/v NaCL solution. One-hundred μL of each dilution was spread in blood agar plates, in order to establish the bacterial concentration of the corresponding suspension. The blood agar plates were incubated at 35-37°C + 5% CO2 for 40-48 hours, after which the number of CFU/ mL grown from each dilution was estimated.
One mL of each dilution (A to F) was inoculated in two different BacT/Alert SA aerobic BCBs (BioMérieux; Durham, North Carolina, USA). In one BCB of each dilution, 1 mL of sterile human blood (leftovers of blood cell packages from the blood bank of our hospital) was added. Finally, serial dilutions of blood (1/2, 1/4, 1/8, 1/16, and 1/32), using BacT/Alert SA aerobic bottle culture medium as a diluting agent, were added to 5 BCBs previously inoculated with 1 mL of dilution C from each strain.
The BCBs were incubated until growth was detected by the automated blood culture instrument, for a maximum of 5 days. When growth was detected in a BCB, a subculture on Columbia agar medium with 5% of sheep blood (blood agar plate) was carried out as a growth control. When no growth was detected after 5 days, the cultures were considered negative and a reseeding was performed as well.
Statistical analysis was carried out using SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA). Categorical variables are reported as proportions and continuous variables as medians with Interquartile Ranges (IQR). Statistical significance was defined as a p-value <0.05.
Results
In blood agar plates created to establish the bacterial concentration of the corresponding suspension, the median number of K. kingae colony in dilution C (the first one in which the growth was not confluent and allowed precise counting) and D were 33 and 2, accounting for the following median (IQR) bacterial concentrations: 330 (26-57) and 20 (2-5) CFU/mL, respectively. Growth was observed in 6 plates with dilution E, but in none with dilution F. Accordingly, estimated mean bacterial concentrations in dilutions A, B, C, D, E, and F were 106-107, 103-104, 102-103, 10-102, 1-10, and 0-1 CFU/mL, respectively.
In non-blood-supplemented BCB, 23 strains grew in BCB seeded with dilution A, only 1 in those seeded with dilution B, and in none of the BCB inoculated with dilutions C to F. In BCB spiked with dilution A suspensions, the median (IQR) Time To Positivity (TTP) was 53.0 (19.5-90.0) hours. All strains grew in blood-supplemented BCB inoculated with dilutions A to D, 22 with dilution E, and only one with dilution F (Figure 1). Increasing TTP was observed with decreasing KK serial dilutions (Friedman rank sum test, p<0.0001). In both blood and non-blood-supplemented BCB, no growth was observed when dilutions from BCB that tested negative by the BactT/ Alert instrument reading were subcultured on blood agar plates.
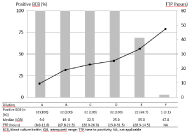
Figure 1: Positivity rates and TTP in blood-supplemented BCBs inoculated
with different KK concentrations (BCB, Blood Culture Bottle; IQR: Interquartil
Range; KK: Kingella kingae; NA: Not Applicable; TTP: Time To Positivity).
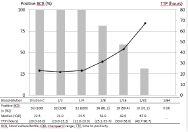
Figure 2: Positivity rates and TTP in BCBs inoculated with KK dilution C in
which different blood concentrations were added (BCB: Blood Culture Bottle;
IQR: Interquartil Range; KK: Kingella Kingae; TTP: Time To Positivity).
In BCB inoculated with dilution C in which decreasing concentrations of human blood were added, all strains grew with blood dilutions 1/2 and 1/4, 26 with dilution 1/8, 19 with dilution 1/16, 10 with dilution 1/32, and none with dilution 1/64 (Figure 2). Increasing TTP was observed again with decreasing blood concentrations (Friedman rank sum test, p=0.001). Time-to-detection was similar among the first 3 serial dilutions and negatively correlated with the concentration of the blood dilutions that were added in the BCB (Spearman’s Rho test; r=-0.632, p<0.0001). The growth curve observed in the BacT/Alert-instrument remained flat up to end of the incubation period in all negative cultures. When BCBs that tested negative by the automated reading were subcultured on blood agar, K. kingae growth was observed in 6, 5, and 5 plates inoculated with dilutions 1/8, 1/16, and 1/32, respectively. This occurred only with the dilution that yielded a first negative BCB result in most cases, but also for 3 strains in which growth was observed in 3, 3, and 2 plates that had been inoculated with BCB containing decreasing concentrations of human blood. The number of isolated colonies was sparse in all cases and, thus, quantitative cultures were not performed.
Discussion
The most efficient method of culturing osteoarticular specimens from pediatric patients with bacterial arthritis or osteomyelitis consist of inoculating the samples in BCB [4,5], which allows the isolation of K. kingae more frequently than in conventional cultures [2,5,6]. The implementation of K. kingae -specific molecular diagnostic methods has increased significantly the detection of this elusive pathogen in recent years [9,10]. In our hospital, we diagnosed 25 cases of osteoarticular infections caused by KK in young children by means of molecular methods from 2012 to 2016; in only 10 (40%) and 3 (7.5%) of these did BCB and conventional cultures yield a positive result, respectively [11]. These data are consistent with other contemporary reports [4,12] and clearly show that a large number of osteoarticular infections in children remain etiologically undiagnosed if only culture methods are performed.
It is known that the concentration of K. kingae in specimens obtained from osteoarticular infections in children is low, ranging from 11 to 300 CFU/mL, [13] and that the microorganism is not usually observed in the Gram stain [2,14,15]. In our own experience, the Gram stain was always negative in a series of children with K. kingae osteoarticular infections [11]. In all likelihood, the low inoculum partly explains the difference in bacterial recovery between conventional cultures and sensitive molecular methods. In this study, K. kingae only grew in non-blood-supplemented BCB when the highest concentrations (dilution A, 106-107 CFU/mL) were inoculated. This inoculum can never be expected in osteoarticular samples.
Conversely, blood-supplemented BCBs universally grew down to dilution D (10-102 CFU/mL) and in 69% of those with dilution E (1- 10 CFU/mL). In clinical practice, with expected KK concentrations around 102 CFU/mL in osteoarticular samples, the microorganism grows in 11-40% of BCB with a positive KK identification in molecular methods (4,11). This estimate is far from both the 0% in non-bloodsupplemented BCBs and the 100% in blood-supplemented BCBs inoculated with similar K. kingae concentrations in our study. In clinical practice, because of the technical difficulties in obtaining, osteoarticular samples are often mixed with patient’s blood at its drawing; those taken with arthrotomy or bone debridement are usually more blood-stained than those obtained with arthrocentesis. We hypothesize that this blood may boost K. kingae growth.
Our results show that the addition of blood to the BCB is critical to enhance the growth of KK: blood-supplemented BCBs universally grew down to K. kingae dilution D (10-102 CFU/mL), the addition of very small volumes of blood led to high rates of positivity (100%, 81%, and 59% with 1/4, 1/8, and 1/16 mL, respectively) with K. kingae dilution C, which is equivalent to those found in osteoarticular samples, and the amount of blood strongly correlated with the BCB TTP. Blood is known to provide hemin and other nutrients and is often used to enrich culture media. However, the specific blood component that enables K. kingae growth remains to be determined.
Adding blood in the BCB together with the osteoarticular specimen could therefore be a practical and inexpensive method to increase its diagnostic efficiency. In our experience, 0.25mL of blood showed a diagnostic efficiency and TTP equivalent to larger volumes; this is a small volume, even for young infants, and could easily be obtained from the patient during the surgical procedure preferably prior to administration of antibiotic therapy. The sparse growth in some of the blood agar plates used as negative controls, alongside the flat growth curve observed in the BacT/Alert System, suggests that the initial inoculum remained in the lag phase in the BCB and never achieved exponential growth.
In conclusion, the addition of human blood was essential to boost the growth of KK in BCB, even when very low numbers of colonies were inoculated. Prospective controlled studies employing skeletal system exudates from actual pediatric patients are needed to determine whether this strategy is useful in the clinical setting.
Acknowledgment
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to disclose.
References
- Yagupsky P, Bar-Ziv Y, Howard CB, Dagan R. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Arch Pediatr Adolesc Med. 1995; 149: 537-540.
- Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis. 2004; 4: 32-41.
- Ferroni A, Al Khoury H, Dana C, Quesne G, Berche P, Glorion C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect. 2013; 19: 822-828.
- Yagupsky P. Kingella kingae: carriage, transmission, and disease. Clin Microbiol Rev. 2015; 28: 54-79.
- Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol. 1992; 30: 1278-1281.
- Moumile K, Merckx J, Glorion C, Pouliquen JC, Berche P, Ferroni A. Bacterial aetiology of acute osteoarticular infections in children. Acta Paediatr. 2005; 94: 419-422.
- Verdier I, Gayet-Ageron A, Ploton C, Taylor P, Benito Y, Freydiere AM, et al. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty four recent pediatric diagnoses. Pediatr Infect Dis J. 2005; 24: 692-696.
- Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007; 26: 377-381.
- Cherkaoui A, Ceroni D, Emonet S, Lefevre Y, Schrenzel J. Molecular diagnosis of Kingella kingae osteoarticular infections by specific real-time PCR assay. J Med Microbiol. 2009; 58: 65-68.
- Lehours P, Freydière AM, Richer O, Burucoa C, Boisset S, Lanotte F, et al. The rtxA toxin gene of Kingella kingae: a pertinent target for molecular diagnosis of osteoarticular infections. J Clin Microbiol. 2011; 49: 1245-1250.
- Gené Giralt A, Ludwig Sanz-Orrio G, Muñoz-Almagro C, Noguera-Julián A. Osteoarticular infections in pediatric patients: The aetiological importance of Kingella kingae. Enferm Infecc Microbiol Clin. 2018; 37: 209-210.
- Juchler C, Spyropoulou V, Wagner N, Merlini L, Dhouib A, Manzano S, et al. The Contemporary Bacteriologic Epidemiology of Osteoarticular Infections in Children in Switzerland. J Pediatr. 2018; 194: 190-196.
- Yagupsky P, Press J. Use of the Isolator 1.5 Microbial Tube for culture of synovial fluid from patients with septic arthritis. J Clin Microbiol. 1997; 35: 2410-2412.
- Dubnov-Raz G, Ephros M, Garty BZ, Schlesinger Y, Maayan-Metzger A, Hasson J, et al. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr Infect Dis J. 2010; 29: 639-643.
- Williams N, Cooper C, Cundy P. Kingella kingae septic arthritis in children: recognising an elusive pathogen. J Child Orthop. 2014; 8: 91-95.