
Special Article - Staphylococcus aureus
J Bacteriol Mycol. 2019; 6(3): 1103.
NaCl Effect on Invasive Staphylococcus aureus Invasion and Immune Responses in the Intestinal Epithelial Cell
Ha J1,2 , Lee S1,2, Kim S1,2, Lee J1,2, Choi Y1, Oh H1, Choi KH3 and Yoon Y1,2*
¹Department of Food and Nutrition, Sookmyung Women’s University, Seoul, Korea
²Risk Analysis Research Center, Sookmyung Women’s University, Seoul, Korea
³Department of Oral Microbiology, Wonkwang University, Iksan, Chonbuk, Korea
*Corresponding author: Yoon Y, Department of Food and Nutrition, Sookmyung Women’s University, Seoul, Korea
Received: March 13, 2019; Accepted: April 09, 2019; Published: April 16, 2019
Abstract
This study investigated the immune responses, which may cause chronic problems through intestinal invasion by Staphylococcus aureus, which is wellknown to cause intoxication rather than invasion, and NaCl effect on the invasion. Five S. aureus strains were cultured and exposed to NaCl concentrations of 0%, 2%, 4%, or 6% supplemented in tryptic soy broth. The surviving cells were isolated by subsequent exposure of the culture on tryptic soy agar containing 0%, 2%, 4%, or 6% NaCl. The resulting cells were subjected to assays evaluating the invasion efficiency into Caco-2 cells, the level of immune responses generated, and the resultant cell viability. For S. aureus strains that invaded Caco-2 cells, the transcriptional analysis for adhesion related genes was conducted by quantitative real-time PCR. Only S. aureus ATCC14458 demonstrated obvious invasion into Caco-2 cells. Invasion efficiency of the strain was influenced by NaCl levels, and expression levels of the cell adhesion related genes were higher at 2%, 4%, and 6% NaCl. In addition, inflammation-related factors were increased. These results indicate that S. aureus ATCC14458 can invade Caco- 2 cells, which may cause infectious illness and other chronic symptoms, and that NaCl increases the extent of Caco-2 cell invasion by expressing specific genes.
Keywords: Inflammation; Invasion; NaCl; Staphylococcus aureus
Introduction
Staphylococcus aureus is usually found in the human nose and skin [1], and thus can be cross-contaminated from humans to foods. S. aureus is weakly competitive in microflora, but it can proliferate in the range of pH 4.0-10.0, Aw >0.86 and even in high salt concentrations up to 10-15% [2]. Thus, S. aureus foodborne illnesses are usually related to salted foods as a result of enterotoxin generation and not by infection [3,4]. NaCl in foods increases the osmotic pressure, which may foodborne pathogens [5]. Biofilm production of S. aureus was increased by exposure to 3% NaCl [6]. In addition, some strains of Listeria monocytogenes exposed to between 2% and 4% NaCl generated slightly thicker biofilms of adherent bacteria [7].
Host cell invasion of bacteria occurs via several mechanisms. Salmonella spp. and Shigella spp. directly manipulate the host cytoskeleton by injecting active proteins intracellularly [8-10]. Yersinia uses a surface protein to bind integrin β1, which is a receptor expressed in the plasma membranes of host cells [11]. L. monocytogenes functions using a similar mechanism; specifically, the internalin A of the bacterium attaches to E-cadherin on the cell surface [10], and the internalin B binds the extracellular domain of c-Met [12]. Unlike these foodborne pathogens, S. aureus produces enterotoxins in foods at 105-106 CFU/g, resulting in intoxication foodborne illnesses [3,13]. If S. aureus is ingested with contaminated food at levels below 105CFU/g, there is no resultant symptom. However, the responses of S. aureus in the intestine have not been elucidated so far.
Therefore, the objective of this study was to identify if S. aureus can invade human intestinal epithelial cells (Caco-2 cell) and stimulate immune responses, and to characterize the effect of NaCl on the invasion process.
Materials and Methods
Cell culture
Caco-2 cells (KCLB 30037.1) were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). The cells were cultured in Eagle’s Minimum Essential Medium (MEM; Gibco, Penrose, Auckland, New Zealand) supplemented with 20% Fetal Bovine Serum (FBS; Gibco) and 1% Penicillin-Streptomycin (PS; Gibco). Media replacement was conducted every 2 to 3 days.
Preparation of inocula
Five S. aureus strains [NCCP10826 (SEC; staphylococcal enterotoxin C), ATCC13565 (SEA), ATCC14458 (SEB), ATCC23235 (SED), and ATCC27664 (SEE)] were cultured in tryptic soy broth (TSB; Becton Dickinson and Company, Franklin Lakes, NJ, USA) at 37°C for 24h, and 0.1mL of the culture was transferred into 10mL TSB. After incubation at 37°C for 24h, the cells were harvested by centrifugation (1,912×g, 4°C, and 15min), washed twice with phosphate buffered saline (PBS, pH 7.4; 0.2g of KH2PO4, 1.5g of Na,2HPO4·7H2O, 8.0g of NaCl, and 0.2g of KCl in 1L of distilled water), and resuspended in PBS. The suspension was diluted in PBS to obtain 4log CFU/mL. The diluent (0.1mL) was then inoculated into 10mL TSB supplemented with 0%, 2%, 4%, or 6% NaCl and incubated overnight at 37°C. The 0.1mL volumes of each culture were plated on tryptic soy agar (TSA; Becton Dickinson and Company) containing 0%, 2%, 4%, or 6% NaCl, to obtain either non-NaCl stress adapted cells or NaCl stress adapted cells. After incubation at 37°C for 24h, 3mL PBS was added over the colonies, and the colonies were scraped with a glass rod. The collected S. aureus cells were then centrifuged (1,912×g, 4°C, 15min), and the pellets were then washed twice with PBS. These bacterial cell suspensions were then adjusted to OD600 = 0.02 for Caco-2 cell invasion assay or adjusted to OD600 = 0.1 for measuring immune responses and cell viability.
Caco-2 cell invasion assay
The 0.5mL volumes of the diluents for five S. aureus strains were inoculated into 4.5mL Eagle’s minimum essential medium (MEM; Gibco, Penrose, Auckland, New Zealand) supplemented with 20% fetal bovine serum (FBS; Gibco), followed by gentle inversions. The 0.1mL volumes of these mixtures were plated on TSA to determine the initial populations of S. aureus. One-milliliter volumes of the mixtures were then inoculated into a monolayer of Caco-2 cells (5×104 cells/mL) in MEM + 20% FBS and incubated in 5% CO2 at 37°C for 2h. The upper layer of MEM + 20% FBS was discarded, and the Caco-2 cells were further incubated in fresh MEM + 20% FBS or fresh MEM + 20% FBS along with 50μg/mL gentamicin to remove the S. aureus cells that were attached on Caco-2 cells in CO2 at 37°C for 2h. After the incubation, the upper layer of the media was discarded, and the Caco-2 cells were washed with Dulbecco’s phosphate buffered saline (DPBS; Welgene, Daegu, Korea) twice. A solution (1mL) of 0.5% Triton X-100 (Sigma-Aldrich Co., St. Louis, State of Missouri, USA) was then added into each well, and the microtiter plate was left on ice for 20min. The resulting suspension (0.1mL) was plated on TSA to enumerate invaded (gentamicin treated group) and attached S. aureus populations (non-gentamicin treated group-gentamicin treated group). The efficiency of S. aureus invasion of Caco-2 cells was calculated by the equation [1] as follows:
Invasion efficiency (%) = [number of S. aureus cells invading Caco-2 cells (CFU/mL)]
× [(initial cell counts of S. aureus {CFU/mL})-1]×100 [1]
Immune response analysis
After evaluating the extent of Caco-2 cell invasion, the invasive strain (S. aureus ATCC14458) was selected. Invasive S. aureus strains and non-invasive S. aureus strains (NCCP10826 and ATCC13565) were subjected to immune response analysis. The S. aureus strains were diluted with MEM to a concentration of 5 × 106 CFU/mL, and 1mL volumes of diluents were inoculated into 5 × 104 cells/mL of Caco-2 cells. They were then incubated in 5% CO2 at 37°C for 24h, followed by centrifugation. After centrifugation, the supernatant was used for immune response analysis with Luminex® extracellular assay (Invitrogen, Carlsbad, CA, USA) in accordance with manufacturer’s protocols.
Cell viability assay
A total of 20μl of the diluents of either invasive S. aureus strain ATCC14458 or the non-invasive S. aureus strains were inoculated into 180μl MEM supplemented with 20% FBS in 5% CO2 at 37°C for 24h. The upper layer of MEM + 20% FBS was discarded, and the Caco-2 cells were further incubated in fresh MEM + 20% FBS along with 100μg/mL gentamicin to extricate the previously attached S. aureus cells from Caco-2 cells at 37°C for 2h. After the incubation, the upper layer of the media was discarded, and the 200μl cell media with 20μl of thiazoly blue tetrazolium bromide (Sigma-Aldrich Co., St. Louis, State of Missouri, USA) solution (5mg/ml in dH2O) was added to each well for 1h. After the incubation, the upper layer of the media was discarded, and 200μl of dimethyl sulfoxide (DMSO; Samchun pure chemical Co., LTD., Pyeongtaek, Korea) was added into each well. Optical density was then measured at 540nm.
Transcriptome analysis
The OD600 value of NaCl stress adapted cells (S. aureus strains ATCC14458, NCCP10826, and ATCC13565) was calibrated to 0.5 with PBS. After centrifuging 1.5mL of the cultures (5,000×g, room temperature, and 5min), the supernatants were discarded, and 200μL of lysostaphin (200μg/mL) (Sigma-Aldrich Co.) was added to the pellets and incubated at 37°C for 20min. By following the manufacturer’s instructions, RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany). The concentration of total RNA was measured using the Epoch Micro-Volume Spectrophotometer System (Bio Tek Instruments, Winooski, VT, USA). Complementary DNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocols. The PCR reaction mixture (25μL) was prepared with the Rotor-Gene SYBR Green PCR Kit (Qiagen) according to the manufacturer’s instructions. The relative expression levels of the genes were analyzed with Rotor-Gene Q software (Qiagen) to compare the expression levels of the genes related to cell adhesion in S. aureus grown in the absence or presence of NaCl. Analysis of the gene expression levels was performed in duplicate per replication, and more than two-fold increases were considered significant [14]. To evaluate the relative expression levels, the primers of the genes are listed in Table 1.
Gene
Primer
Sequence (5'®3')
16s RNA
16s RNA-F
GAG GGT CAT TGG AAA CTG GA
16s RNA-R
CAT TTC ACC GCT ACA CAT GG
icaA
icaA-F
CGC AGC AGT AGT TCT TGT CG
icaA-R
TGA CCA TGT TGC GTA ACC AC
icaB
icaB-F
AAG CAG TCA CTC CGA ACT CC
icaB-R
ATG GAA TCC GTC CCA TCT CT
icaC
icaC-F
TGG AAC GTT ACC AGC TTT TCA
icaC-R
AAT GCG TGC AAA TAC CCA AG
icaD
icaD-F
TTG AAA CTA TGG GCA TTT TCG
icaD-R
CAC GAT TCT CTT CCT TTC TGC
fnbA
fnbA-F
CTT TGG CAG GTG GTA CTG GT
fnbA-R
GAG CCA GAA ACT CCA ACA CC
Table 1: Oligonucleotide primers used in the quantitative real-time PCR analysis.
Abstract
The experiment was repeated twice with four samples per repeat (n=8). The experimental data for the quantitative analysis was analyzed with the general linear procedure of SAS® version 9.2 (SAS Institute Inc., Cary, NC, USA). The mean comparisons were performed by a pairwise t-test at a = 0.05.
Results
Caco-2 cell invasion and immune response
Of the five S. aureus strains examined, S. aureus ATCC14458 had higher (p‹0.05) adhesion efficiency (1.887-6.443 %) on Caco-2 cells than the other strains (0.000-0.029 %) (Table 2). In addition, only S. aureus ATCC14458 (3.968-9.097 %) showed obvious Caco-2 cell invasion compared to the other strains (0.005-0.263 %), and the Caco-2 cell invasion efficiency of the strain was increased (p‹0.05) as the NaCl concentration that S. aureus ATCC14458 was exposed to increased (Table 3). To confirm the inflammation, S. aureus ATCC14458 was subjected to immune response analysis and the levels of 40 cytokines, chemokines, and growth factors were measured. The cytokines, chemokines, and growth factors generated by S. aureus ATCC14458 or non-invasive strains (NCCP10826 and ATCC13565) were measured. Especially, IFNγ was increased (p‹0.05) in invasive S. aureus strain by protective inflammatory reaction in Caco-2 cell. Interestingly, the Caco-2 cells inoculated with invasive S. aureus strain exhibited higher (p‹0.05) production of Fibroblast Growth Factor-2 (FGF-2), Fractalkine (FKN), IL-12 (p70), monocyte chemoattractant protein-3 (MCP-3), platelet derived growth factor-AB/BB (PDGFAB/ BB), and Vascular Endothelial Growth Factor (VEGF) than noninvasive strains (Table 4). Although it was observed that S. aureus ATCC14458 can elicit the production of inflammatory cytokines, chemokines, and growth factors the concentration of NaCl employed did not affect the levels of cytokines secreted (Table 5).
Strain
NaCl concentration (%)
0
2
4
6
10826
0.004±0.000 Ab
0.002±0.000 Ab
0.001±0.000 Ab
0.001±0.000 Ab
13565
0.011±0.012 Ab
0.015±0.011 Ab
0.029±0.040 Ab
0.020±0.023 Ab
14458
1.887±1.124 Ca
6.443±3.153 Aa
4.010±1.908 Ba
3.269±0.874 BCa
23235
0.005±0.001 Ab
0.012±0.002 Ab
0.001±0.001 Ab
0.001±0.000 Ab
27664
0.015±0.020 Ab
0.015±0.006 Ab
0.000±0.000 Ab
0.005±0.007 Ab
A-CMeans within the same row with different superscript letters are significantly different (p<0.05).
a-bMeans within the same column with different superscript letters are significantly different (p<0.05).
Table 2: Adhesion efficiency (mean ± standard deviation) of Staphylococcus aureus strains NCCP10826, ATCC13565, ATCC14458, ATCC23235, and ATCC27664, which were exposed to tryptic soy agar plus 0, 2, 4, and 6% NaCl.
Strain
NaCl concentration (%)
0
2
4
6
10826
0.005±0.000 Ab
0.017±0.004 Ab
0.005±0.000 Ab
0.037±0.010 Ab
13565
0.025±0.015 Ab
0.035±0.001 Ab
0.010±0.008 Ab
0.097±0.074 Ab
14458
3.968±0.834 Ca
5.480±3.281 BCa
5.847±1.590 Ba
9.097±1.241 Aa
23235
0.174±0.052 Ab
0.216±0.046 Ab
0.099±0.061 Ab
0.191±0.132 Ab
27664
0.237±0.271 Ab
0.263±0.079 Ab
0.089±0.061 Ab
0.157±0.022 Ab
A-CMeans within the same row with different superscript letters are significantly different (p<0.05).
a-bMeans within the same column with different superscript letters are significantly different (p<0.05).
Table 3: Invasion efficiency (mean ± standard deviation) of Staphylococcus aureus strains NCCP10826, ATCC13565, ATCC14458, ATCC23235, and ATCC27664, which were exposed to tryptic soy agar plus 0, 2, 4, and 6% NaCl.
Group
Analyte name
MEM blank
MEM
ATCC14458
NCCP10826
ATCC13565
(pg/ml)
+cell blank
(pg/ml)
Cytokines
Flt-3 Ligand
2
7
64±10 A
10±10 B
6±8 B
G-CSF
4
6
57±0 A
13±1C
25±3 B
GM-CSF
0
0
38±9 A
1±1 B
1±1 B
IFNa2
0
0
60±2 A
5±6 B
2±1 B
IFNγ
0
0
107±10 A
6±5 B
1±2 B
IL-1a
0
0
1±0 A
0±0 B
0±0 B
IL-1β
0
1
6±0 A
0±0 B
0±1 B
IL-1γa
1
1
17±3 A
3±1 B
1±1 B
IL-2
0
0
6±1 A
0±0 B
0±0 B
IL-3
0
0
43±4 A
1±1 B
1±1 B
IL-4
2
5
16±2 A
4±2 B
8±2 B
IL-5
0
1
2±0 A
1±0 A
1±1 A
IL-6
0
0
32±1 A
3±1 B
2±1 B
IL-7
0
0
9±0 A
2±3 A
3±4 A
IL-8
1
18
23±1 C
64±0 A
49±2 B
IL-9
0
0
24±3 A
2±0 B
2±0 B
IL-10
0
0
6±3 B
0±0 A
1±0 A
IL-12(p40)
0
0
56±3 A
1±1 B
0±0 B
IL-12(p70)
2
3
256±6 A
12±3 B
14±4 B
IL-13
0
0
6±1 A
0±0 B
0±0 B
IL-15
1
1
10±1 A
2±0 B
1±0 B
IL-17
0
0
25±0 A
1±0 B
2±1 B
TNFa
0
1
6±0 A
1±0 B
0±0 B
TNFβ
0
0
2±3 A
0±0 A
1±1 A
Chemokines
Eotaxin
9
5
55± 1 A
17±4 B
20±4 B
Fractalkine (FKN)
5
0
135±11 A
25±21 B
61±3 B
IP-10
0
84
107±4 B
231±10 A
222±41 A
MCP-1
1
6
10±2 A
12±0 A
10±2 A
MCP-3
8
16
145±11 A
24±4 B
30±11 B
MDC
0
3
14±2 A
6±0 B
0±0 C
MIP-1a
6
4
28±2 A
5±7 B
0±0 B
MIP-1β
0
0
23±1 A
0±0 B
5±3 B
RANTES
3
4
8±0 A
6±1 A
3±4 A
Growth factors
EGF
1
1
27±2 A
6±4 B
5±3 B
FGF-2
9
7
382±22 A
43±11 B
32±6 B
GRO
0
269
591±54 B
962±17 A
854±94 A
PDGF-AA
0
235
112±12 B
161±1 AB
169±28 A
PDGF-AB/BB
13
8
236±37 A
20±4 B
17±3 B
TGFa
0
0
2±0 A
0±0 B
0±0 B
VEGF
0
64
334±32 A
82±44 B
0±0 B
A-CMeans with the same row with different superscript letters are significantly different (p<0.05).
Table 4: Cytokines, chemokines, and growth factors stimulated by invasive Staphylococcus aureus strain (ATCC14458), and non-invasive S. aureus strains (NCCP10826 and ATCC13565).
Group
Analyte name
MEM blank
MEM
0%
2%
4%
6%
(pg/ml)
+cell blank
(pg/ml)
Cytokines
Flt-3 Ligand
0
0
38±13 A
23±3 A
29±0 A
31±1 A
G-CSF
0
0
5±7 A
0±0 A
0±0 A
2±3 A
GM-CSF
3
3
20±8 A
11±2 A
12±0 A
13±0 A
IFNa2
0
0
3±3 A
0±0 A
2±1 A
1±0 A
IFNγ
0
0
13±5 AB
8±1 B
17±2 A
18±1 A
IL-1a
0
0
2±1 A
0±0 A
2±1 A
1±1 A
IL-1β
2
2
13±5 A
8±0 A
9±0 A
8±0 A
IL-1γa
0
0
32±16 A
20±0 A
16±6 A
19±5 A
IL-2
0
0
22±9 A
10±1 A
13±1 A
12±1 A
IL-3
1
1
14±0 A
5±0 B
5±1 B
5±1 B
IL-4
0
0
3±4 A
1±0 A
1±1 A
1±1 A
IL-5
1
1
1±0 A
1±0 A
1±0 A
1±0 A
IL-6
0
0
11±4 A
5±0 B
6±0 AB
6±0 AB
IL-7
1
1
2±0 A
1±1 A
2±0 A
2±0 A
IL-8
3
6
11±1 A
9±2 A
13±0 A
15±0 A
IL-9
2
2
20±10 A
11±1 A
13±0 A
12±0 A
IL-10
0
0
0±0 A
0±0 A
0±0 A
0±0 A
IL-12(p40)
0
0
108±44 A
58±10 A
70±8 A
72±1 A
IL-12(p70)
2
2
82±25 A
34±3 B
45±4 B
51±0 AB
IL-13
2
2
3±0 A
2±0 A
3±0 A
3±0 A
IL-15
4
4
12±2 A
9±1 A
9±1 A
9±0 A
IL-17
1
0
8±1 A
5±2 A
10±1 A
11±1 A
TNFa
0
0
1±1 A
0±0 A
0±0 A
0±0 A
TNFβ
0
0
0±0 A
0±0 A
0±0 A
0±0 A
Chemokines
Eotaxin
9
7
49±10 A
34±1 A
41±3 A
42±0 A
Fractalkine (FKN)
0
0
136±47 A
77±19 A
126±16 A
147±46 A
IP-10
5
27
31±2 A
26±1 A
29±1 A
31±4 A
MCP-1
0
0
9±1 A
6±0 A
8±1 A
7±0 A
MCP-3
9
4
56±12 A
32±3 B
43±3 AB
47±2 AB
MDC
7
5
22±5 AB
16±2 B
28±4 A
30±3 A
MIP-1a
8
5
16±12 A
10±3 A
19±2 A
19±2 A
MIP-1β
0
0
14±4 A
7±0 B
9±1 AB
8±1 AB
RANTES
5
4
6±0 A
6±0 A
5±0 A
6±1 A
Growth factors
EGF
3
1
60±25 A
37±3 A
46±3 A
50±0 A
FGF-2
0
0
777±215 A
457±47 B
529±24 AB
549±18 AB
GRO
0
134
214±13 A
191±4 A
198±21 A
226±18 A
PDGF-AA
0
68
37±0 A
32±2 A
32±1 A
34±2 A
PDGF-AB/BB
0
18
156±34 A
99±4 B
155±9 A
185±14 A
TGFa
1
1
2±1 A
1±0 A
2±0 A
2±0 A
VEGF
0
38
103±24 AB
66±3 B
104±0 A
115±10 A
A-BMeans with the same row with different superscript letters are significantly different (p<0.05).
Table 5: Cytokines, chemokines, and growth factors stimulated by invasive Staphylococcus aureus strain (ATCC14458) experienced 0, 2, 4, and 6% NaCl.
Caco-2 cell viability analysis
To evaluate if invasive S. aureus can cause cytotoxicity in Caco-2 cells, an MTT assay was conducted [15]. The MTT assay demonstrated that there is no difference in cytotoxicity among the strains (p>0.05), regardless of NaCl concentrations (Figure 1). This result indicates that even though invasive S. aureus strains invade intestinal Caco-2 cell, the actual process of invasion itself does not cause cytotoxicity. Accordingly, we hypothesize that while the inflammation and other clinical symptoms immediately ensuing occur without resulting in intestinal cell damage, the symptoms may gradually develop to trigger disease in the long term.
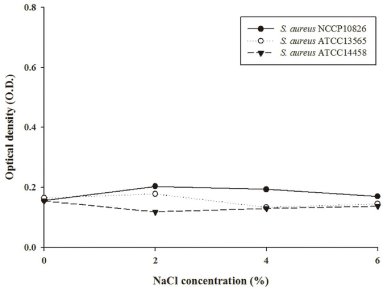
Figure 1: Cytotoxicity of NaCl (0%, 2%, 4%, and 6%)-adapted invasive
Staphylococcus aureus strain (ATCC14458), and non-invasive S. aureus
strains (NCCP10826 and ATCC13565) on Caco-2 cells.
Transcriptome analysis
To evaluate the correlation between the genes influenced by NaCl concentrations and S. aureus Caco-2 cell invasion, the expression levels of icaA, icaB, icaC, icaD, and fnbA were analyzed using qRTPCR after S. aureus was exposed to 0%, 2%, 4%, or 6% NaCl. icaA, icaB, icaC, and icaD are the genes included in ica operon, which plays a role in polysaccharide intercellular adhesion [16]. fnbA is the gene encoding fibronectin-binding protein, which mediates bacterial adherence and coagulase [17]. Notably, the expression levels of all genes tested were higher in the invasive S. aureus strain, as compared to those in the non-invasive strains. The expression levels were also increased at higher NaCl concentration in S. aureus ATCC14458. Especially, the expression level of fnbA was increased by more than two-fold at 2%, 4%, and 6% NaCl, compared to that of 0% NaCl (p‹0.05) (Figure 2A). This result suggests that the NaCl in food upregulates the ica operon and fnbA of invasive S. aureus strains, and that its attachment is regulated by polysaccharide intercellular adhesion, which may increase intercellular invasion. However, the expression levels of these genes were not increased in non-invasive S. aureus strains (Figure 2B and 2C)
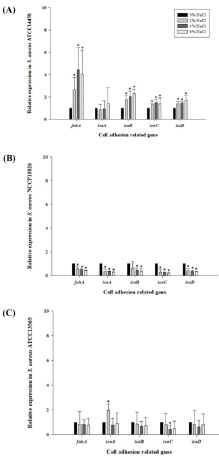
Figure 2: Relative expression of cell adhesion related genes after invasive
Staphylococcus aureus strain (ATCC14458; A) and non-invasive S. aureus
strains (NCCP10826; B and ATCC13565; C) were exposed to 0%, 2%, 4%,
and 6% NaCl. *: Statistical significance composed to 0% NaCl at a=0.05.
Discussion
Caco-2 cell invasion and immune response data indicates that even though S. aureus is known to cause foodborne illnesses due to the consumption of enterotoxins produced by the pathogens in food [3], S. aureus ATCC14458 strain may cause infection in intestines, resulting in inflammation. NaCl in food may increase its infection because the genes are sensitively upregulated in S. aureus ATCC14458, which could be a major cause making the strain invasive. The Caco- 2 cells inoculated with invasive S. aureus strains exhibited higher production of some cytokines, chemokines, and growth factors as a part of protective mechanisms in the host cell [18,19]. FGF- 2 plays a role in protecting cells from necrosis [20]. Further, FKN was secreted under inflammatory conditions in endothelial cells to coordinate leukocyte trafficking and thus, FKN expression is related to various inflammatory diseases [21,22]. In addition, it promotes the progression of cancer by supporting tumor angiogenesis [23]. The expression level of PDGF is low or undetectable in normal adult tissues, but its level is increased following tissue damage [24]. IL-12, produced by macrophages and B cells is also a powerful immunopotentiating agent. When IL-12 was administered to BALB/c mice infected with Mycobacterium tuberculosis, their survival time increased and there was a concomitant reduction in the number of bacteria present [25]. Moreover, IL-12 increased resistance to several intracellular pathogens in murine models. IL-12 has about seven times higher expression in breast tumor tissue as compared to normal tissue, and it plays an important role in the control of early stages of breast cancer [26] and inflammation response [27]. VEGF is known to play an important role as a stimulant in cancer angiogenesis [28]. Also, VEGF itself is not inflammatory, but it modulates the overall immune response associated with inflammation [29]. Taken together, S. aureus ATCC14458 may invade Caco-2 cells, resulting in the increased production of cytokines related to inflammation, chemokines, and growth factors. However, we found no differences in cell viabilities between invasive and non-invasive strains. Coussens and Werb [30] suggested that long-term inflammation in intestine may cause carcinogenesis. Thus, it can be suggested that although S. aureus invasion in intestine did not cause acute symptoms, but the inflammation caused by S. aureus invasion may cause chronic problems in long term. These results indicate that intake of S. aureus likely causes infection and potentially promotes microenvironmental conditions that are conducive to the development of various diseases. Therefore, further study is needed to elucidate the correlation between the S. aureus invasion and chronic disease development.
In conclusion, although S. aureus has been well established as a cause of intoxication foodborne illnesses, a S. aureus strain having high ica operon and fnbA gene expression can be ingested through food consumption and invade intestinal epithelial cells, and this may induce inflammation and chronic diseases in long term. In addition, the invasive efficiency is increased as the NaCl concentrations that S. aureus are exposed to in food increase.
Acknowledgement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2012R1A1A1014562).
References
- Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008; 322: 207-228.
- Jones EM, Bowker KE, Cooke R, Marshall RJ, Reeves DS, MacGowan AP. Salt tolerance of EMRSA-16 and its effect, on the sensitivity of screening cultures. J Hosp Infect. 1997; 35: 59-62.
- Le-Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003; 2: 63-76.
- USFDA (United States Food and Drug Administration). Bad bug book: Foodborne pathogenic microorganisms and natural toxins handbook. 2004. New York: Valley Stream.
- Choi K, Yoon Y. The effects of sodium chloride on the physiological characteristics of Listeria monocytogenes. Korean J Food Sci Ani Resour. 2013; 33: 395-402.
- Kennedy CA, O’Gara JP. Contribution of culture media and chemical properties of polystyrene tissue culture plates to biofilm development by Staphylococcus aureus. J Med Microbiol. 2004; 53: 1171-1173.
- Jensen A, Larsen MH, Ingmer H, Vogel BF, Gram L. Sodium chloride enhances adherence and aggregation and strain variation influences invasiveness of Listeria monocytogenes strains. J Food Protect. 2007; 70: 592-599.
- Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998; 93: 815-826.
- Isberg RR, Van Nhieu GT. Two mammalian cell internalization strategies used by pathogenic bacteria. Annu Rev Genet. 1994; 28: 395-422.
- Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996; 84: 923-932.
- Isberg RR, Leong JM. Multiple β 1 chain integrins are receptors for invasion, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990; 60: 861-871.
- Shen Y, Naujokas M, Park M, Ireton K. InlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000; 103: 501-510.
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000; 13: 16-34.
- Kazama H, Yonehara S. Oncogenic K-Ras and basic fibroblast growth factor prevent Fas-mediated apoptosis in fibroblasts through activation of mitogenactivated protein kinase. J Cell Biol. 2000; 148: 557-566.
- Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009; 113: 963-972.
- White GE, Greave DR. Fractalkine: One chemokine, many functions. Blood. 2009; 113: 767-768.
- D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: Why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010; 14: 207-219.
- Biarc J, Nguyen IS, Pini A, Gossé F, Richert S, Thiersé D, et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S. bovis). Carcinogenesis. 2004; 25: 1477-1484.
- Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996; 98: 945-953.
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991; 253: 1129-1132.
- Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995; 155: 2515-2524.
- Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 1996; 56: 2013-2016.
- Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999; 59: 1592- 1598.
- Garner MR, James KE, Callahan MC, Wiedmann M, Boor KJ. Exposure to salt and organic acids increases the ability of Listeria monocytogenes to invade Caco-2 cells but decreases its ability to survive gastric stress. Appl Environ Microbiol. 2006; 72: 5384-5395.
- DeRisi JL, Lyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997; 278: 680-686.
- Seidl K, Zinkernagel AS. The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J Microbiol Methods. 2013; 92: 307-309.
- Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998; 70: 83-243.
- Nuryastuti T, van der Mei HC, Busscher HJ, Iravati S, Aman AT, Krom BP. Effect of cinnamon oil on icaA expression and biofilm formation by Staphylococcus epidermidis. Appl Environ Microbiol. 2009; 75: 6850-6855.
- Proescholdt MA, Heiss JD, Walbridge S, Mühlhauser J, Capogrossi MC, Oldfield EH, Merrill MJ. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol. 1999; 58: 613-627.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420: 860- 867.