
Special Article - Mycology
J Bacteriol Mycol. 2019; 6(3): 1104.
Fatal Hematogenous Cryptococcosis in Apparently Immunocompetent Patient: Case Report
Santiago Rocha AP1*, Nunes M1, Inácio CP1, Diniz MV2, Lima Neto RG3, Gonçalves de Godoy MM4 and Neves RP1
1Department of Mycology, Federal University of Pernambuco (UFPE), Av. da Engenharia, s/n, Cidade Universitária, Recife-PE, CEP: 50740-550. Recife-PE, Brazil
2Clinical Hospital, Federal University of Pernambuco (UFPE), Av. da Engenharia, s/n, Cidade Universitária, Recife-PE, CEP: 50740-550. Recife-PE, Brazil
3Department of Tropical Medicine, Federal University of Pernambuco (UFPE), Av. da Engenharia, s/n, Cidade Universitária, Recife-PE, CEP: 50740-550. Recife-PE, Brazil
4Intensive Medicine Unit of Hospital das Clínicas, Federal University of Pernambuco (UFPE), Av. da Engenharia, s/n, Cidade Universitária, Recife-PE, CEP: 50740-550. Recife-PE, Brazil
*Corresponding author: Ana Paula Santiago Rocha, Department of Mycology, Federal University of Pernambuco (UFPE), Av. da Engenharia, s/n, Cidade Universitária, Recife-PE, CEP: 50740-550. Recife-PE, Brazil
Received: April 09, 2019; Accepted: April 29, 2019; Published: May 06, 2019
Abstract
Cryptococcosis is an opportunistic fungal infection caused by encapsulated and cosmopolitan yeasts, of which the causative agents of the disease are recognized as the complex of Cryptococcus neoformans species. This mycosis can have subacute or chronic clinical profile, and can be fatal, especially in immunocompromised individuals such as patients with HIV/AIDS, neutropenic individuals and transplant recipients. Here we report a fatal case of hematogenous cryptococcosis in a non-immunosuppressed individual. The patient, a 53-year-old adult male, was hospitalized in the ICU of a hospital in Recife, Pernambuco, Brazil. The patient died 48 hours after admission to the ICU. The etiological agent was identified by an automated testing system (BD PhoenixTM) and molecular taxonomy. The cause of the infection was diagnosed as Cryptococcus neoformans. Hematogenous cryptococcosis or cryptococcemia is a systemic mycosis that mainly affects immunocompromised individuals, and is identified by a blood culture positive for yeasts of the genus Cryptococcus. It is a rare disease compared to the other forms of clinical presentation, especially when the patient does not have a clinical condition compatible with immunosuppression.
Keywords: Cryptococcus Neoformans; Meningitis; Hematogenous Cryptococcosis; Immunocompromised
Introduction
Cryptococcosis is an opportunistic fungal infection caused by encapsulated and cosmopolitan yeasts of which the causative agents of the disease are recognized as the complex of Cryptococcus neoformans species, grouping the commonly known varieties C. neoformans var grubii and C. neoformans var neoformans and serotypes (A, D and AD), and the complex of species C. gattii, with serotypes B and C [1,2]. In Latin America, the study of this mycosis has been frequent, due to the high morbidity and mortality rates with more than 5,000 individuals affected with cryptococcal meningitis each year and 2,400 attributable annual deaths in Latin America alone [2].
This mycosis can have a subacute or chronic clinical profile, and is potentially fatal [3]. It can affect both apparently immunocompetent individuals and those who suffer from grave conditions, such as patients with HIV/AIDS, neutropenic individuals, transplant recipients, people with hematological malignancies and those undergoing prolonged therapy with corticosteroids [4-7]
Cryptococcosis often occurs due to the inhalation of viable fungal propagules, found in various substrates, such as decomposing organic matter, contaminated soil, and bird droppings, especially of pigeons. After inhalation, Cryptococcus presents the lungs as the primary focus of infection, where it can be phagocytosed by alveolar macrophages [8,6,9]. This disease is commonly reported afflicting the central nervous system, but also the kidneys, bones and skin [10,9].
While cryptococcosis of the central nervous system is the most frequent clinical form, hematogenic cryptococcosis in immunocompetent patients is rare, since it is considered an uncommon form of clinical presentation of the disease, and clinical manifestations are non-specific [4].
Due to a higher prevalence of bacterial infections and empirically prescribed treatments, such factors often contribute to the delay in the diagnosis of fungal infections, favoring a worse prognosis of the disease [11]. We report here a case of hematogenic cryptococcosis in a patient apparently immunocompetent, as a clinical-epidemiological alert in relation to this disease. We report here a case of hematogenic cryptococcosis in a patient apparently immunocompetent, as a clinical-epidemiological alert regarding the etiological agent of this disease.
Case Presentation
The patient was a 53-year-old unemployed man with dark skin. He had a history over the preceding three months of generalized myalgia, anorexia, steady weight loss (+/-20kg) and severe depression. Two weeks before being hospitalized, his clinical condition had become worse, with fever, dry cough, episodes of disorientation and psychomotor agitation. There was a recent diagnosis of systemic arterial hypertension. He stated he was a nonsmoker. At the start of hospitalization, hematuria was noted, with urine test results suggestive of infection. In response, intravenous empirical antibiotic therapy was started with ciprofloxacin, to treat sepsis with urinary focus. The patient continued with to constant fever and central nervous system alterations. On the fourth day after admission, he was transferred to the intensive care unit due to deteriorating clinical profile, with important dyspnea and hypoxemia, requiring mechanical breathing assistance due to severe acute respiratory insufficiency. New samples were collected (blood, urine and tracheal secretion) and the antimicrobial treatment regimen was modified, with administration of ceftriaxone and clindamycin, for respiratory tract infection due to probable bronchoaspiration. The results of the laboratory tests conducted at the moment of admission indicated leukocytosis (leukocytes = 13,700 cells/mm3 with 88.2% neutrophils/ reference value = 4.000-11.000/55-60 %), hemoglobin = 16g/dl (reference value = 14-18 g/dl); hematocrit = 47.5% (reference value = 42-52 %), urea = 98mg/dL (reference value = 19-49 mg/dL), creatinine = 0.9mg/dL (reference value = 0,7-1.3 mg/dL), aspartate aminotransferase = 51U/L (reference value = 15-46 U/L), alanine aminotransferase = 57 U/L (reference value = 9-72 U/L), lactate dehydrogenase = 619 U/L (reference value = 313-613 U/L), creatine phosphokinase = 195U/L (reference value = 30-190 U/L) and albumin = 2.6g/dL (reference value = 3,5-4,7 g/dL). The HIV and urine tests were negative. Computerized tomography of the thorax was carried out, revealing evidence of thrombus in the arterial segment of the lower right lobe, suggestive of pulmonary thromboembolism; and parenchymatous opacities in the lower lobes, some of them centroacinar with branched pattern, especially in the upper segment of the lower right lobe. Tomography of the abdomen revealed slightly heterogeneous texture of the liver, without signs of chronic hepatic disease. No other significant alterations were observed. Because of the deteriorating clinical profile and imaging results, bacilloscopy for BK of the tracheal secretion was requested and full intravenous anticoagulation therapy was initiated with enoxaparin. Forty-eight hours after transfer to the ICU, the patient died, before received the microbiological results of the blood, urine and tracheal secretion cultures. The blood from the catheter was collected aseptically (following biosecurity standards) in Vacutainer® tubes with EDTA by venous puncture and was immediately transferred to flasks containing Brain Heart Infusion (BHI) medium. The flasks were then placed in a Bact/ALERT® incubator and the presence Cryptococcus neoformans was identified with a BD PhoenixTM automated system. Blood samples were also sent to the Medical Mycology Laboratory of Pernambuco Federal University (UFPE), where sheets contrasted with India ink were examined and the presence was observed of encapsulated yeasts (Figure 1). Subsequently, blood samples were seeded in Duplicate on Sabouraud Dextrose Agar (Difco) plus 50mg/L of chloramphenicol in Petri dishes, which were kept at temperatures of 30°C and 37°C for up to 15 days for isolation of the etiological agent. Finally, confirmation was performed by molecular taxonomy, where DNA from the yeast was extracted and submitted to sequencing of the D1-D2 domain of rDNA [12]. The primers used were NL1 (5’ - GCATATCAATAAGCGGAGGAAAAG-3’) and NL4 (5’ - GGTCCGTGTTTCAAGACGG - 3’). The PCR cycling consisted of initial denaturing at 95°C for 5min, followed by 35 denaturing cycles at 95°C for 30s each, annealing at 57°C for 30s, extension at 72°C for 30s and final extension at 72°C for 10min. At the end of the process, the samples were purified with the GeneJET PCR purification kit (Fermentas, UK) and sequenced by the sequencing platform of the Central Laboratory of the Center for Biological Sciences of UFPE. The isolate in question was identified as Cryptococcus neoformans, a sample of which is now stored in the culture collection of UFPE under URM7730.
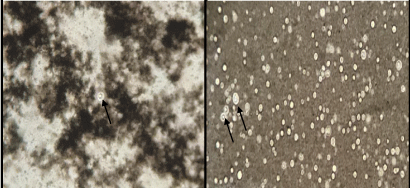
Figure 1: Direct microscopic images of blood cultures stained with India
ink, revealing budding and encapsulated yeast cells (arrows). Magnification:
400x.
Discussion
It is estimated that C. neoformans is responsible for more than a million cases of cryptococcosis a year worldwide [13]. From those, Brazil and Colombia were the countries with the highest incidence, between 1,001 to 2,500 cases, followed by Argentina and Mexico with an incidence of 501 to 1,000 cases [14]. In particular, an average annual incidence of 4.5 cases of meningeal cryptococcosis per 106 inhabitants was reported in the general population of the state of Rio de Janeiro, Brazil [15,2].
Generally, the species C. neoformans affects hosts having some type of immunosuppression, while C. gattii preferentially infect immunocompetent hosts [16,17,6]. In the case reported here, the patient did not present an apparent immunosuppression condition that can explain the infection by C. neoformans, an uncommon change in the epidemiological profile of the infection.
No predilection for the disease was observed regarding age, sex or profession. Instead, the most common predisposing factor is immunosuppression-involving alteration of cell function in defense of the organism [4]. The signs and symptoms vary depending on the infection site. General symptoms like fever, headache, malaise and alterations in the level of consciousness are unspecific, common in sepsis with varied origins. It is therefore necessary to increase the sensitivity in diagnosing suspected infections of the central nervous system, in the absence of classic symptoms of meningitis such as neck stiffness, headaches and signs of meningeal irritation [18]. These symptoms are very specific for meningitis, but are not always present. Indeed, in cases of fungal meningitis caused by species of Cryptococcus, they are absent 75% of the time [19]. Early examination of the Cerebrospinal Fluid (CSF) is of great importance, so it is necessary to be wary of the possibility of infection by these fungi, to avoid delay in diagnosis and treatment and reduce the mortality of these patients. In the case reported here, the CSF was not examined due to lack of suspicion and the rapid clinical deterioration of the patient.
The primary infection generally has focus in the lungs, causing severe respiratory problems such as pulmonary insufficiency [20]. Hematogenous cryptococcosis, or cryptococcemia, is a systemic mycosis that mainly affects immunocompromised individuals. It is diagnosed by blood culture positive for yeasts of the genus Cryptococcus [21,22]. It is a rare disease condition in immunocompetent individuals compared to other clinical forms [22,23]. Although the tests performed in the case reported here showed negative serology for HIV, absence of Mycobacterium infection, malignant diseases and diabetes mellitus, but C. neoformans was isolated from the blood. In this case, a previous respiratory problem might have been a factor in the development of cryptococcemia, possibly after inhaling encapsulated yeast spores [24,25].
The mycological diagnosis of cryptococcosis is confirmed by the isolation of the fungus in culture medium, histopathological exams with specific stains and serological tests. Biological samples such as CSF, urine, tissue fragments, aspirated from lesions and other respiratory tract samples may directly or indirectly demonstrate the presence of Cryptococcus spp. Currently, molecular techniques have been increasingly used, due to their ability to classify species within genetic complexes and the different profiles of drug resistance of choice [26,27].
Consensus exists regarding treatment of cryptococcosis. The “Practice Guidelines for the Management of Cryptococcal Disease” establish protocols for management of individuals affected by cryptococcosis. For HIV patients as non-HIV, is recommended therapy with amphotericin B (AmB) deoxycholate (0.7-1.0 mg/kg per day) with fluocytosin (100mg/kg per day). For non-transplant patients, the recommended treatment is liposomal amphotericin B (3-4 mg/kg per day) associated with fluocytosin (100mg/kg per day). In case of cryptococcemia where the patient does not have risk factors for immunosuppression, the recommended therapy is fluconazole (400mg [6mg/kg] per day orally) for 6-12 months [28,29].
Although the sudden and early death of the patient did not allow the implementation of specific antifungal treatment, we emphasize the importance of mycological diagnosis associated with antibacterial therapies and continuous evaluations of the presence of microorganisms in the blood through routine blood cultures. Here we report the case of a patient suffering from sepsis with probable pulmonary focus, followed by hematogenous infection, where the clinical complications combined with the failure to suspect fungal infection led to tardy diagnosis and early death, being the definitive route of infection, in this case, apparently unknown.
Acknowledgement
We thank to Hospital Agamenon Magalhães.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Authors’ Contributions
All authors contributed substantially to the design of the study and analysis and interpretation of the data. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Ethics approval
This study was conducted after approval by the hospital’s committee on ethical research with human.
Informed consent
Informed written consent was obtained from the patient prior to publication of the case details.
References
- Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, Bicanic TA, et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere. 2017.
- Firacative C, Lizarazo J, Illnait-Zaragozí MT, Castañeda E, Latin American Cryptococcal Study Group. The status of cryptococcosis in Latin America. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2018; 113: e170554.
- AlMutawa F, Leto D, Chagla Z. Disseminated cryptococcal disease in Non- HIV, non-transplant patient. Case Reports in Infectious Diseases. 2016.
- Nath R, Laskar B, Ahmed J, Subhalakshmi DS, Timung L, Saikia L. Cryptococcus neoformans var. grubii: Infection in HIV seronegative patients from northeast India: Report of two cases with review of Literature. Mycopathologia. 2016; 181: 315-321.
- Oliver NT, Lazarus DR, Hamill RJ, Restrepo A. Isolated Cryptococcal neoformans Pleuritis in a Lung Cancer Patient: Case Report and Review of the Literature. Curr Trop Med Rep. 2016; 3: 176-180.
- Inaba A, Okada A, Yoshida T, Itoyama S, Nakai T, Hisada T, Takano H. Disseminated Cryptococcosis with Rapidly Growing Lung Nodules in an Endstage Renal Disease Patient. Intern Med. 2017; 56: 377-380.
- Frola C, Guelfand L, Blugerman G, Szyld E, Kaufman S, Cahn P, et al. Prevalence of cryptococcal infection among advanced HIV patients in Argentina using lateral flow immunoassay. PLoS ONE. 2017; 12: e0178721.
- Neves RP, Lima-Neto R.G, Silva VKA, Leite MC, Santos FAG, Macêdo DPC. Cryptococcus laurentii fungaemia in a cervical cancer patient. The Brazilian Journal of Infectious Diseases (Impresso). 2015; 19: 660-663.
- Hayashida MZ, Seque CA, Pasin VP, Enokihara MMSS, Porro AM. Disseminated cryptococcosis with skin lesions: Report of a case series. An Bras Dermatol. 2017; 92: 69-72.
- McKinney JL, Cerio D, Loghmanee C, Pinho P, Gomes R, Patel M, et al. Surgical Management of Primary Cutaneous Cryptococcosis after Failed Medical ManagementMicrosurg. 2015; 7: 116-118.
- Kandathil JP, Rupinder K, Mohan S, Kanwal M, Navjot S, Anna M. Disseminated cryptococcosis presenting with generalized lymphadenopathy. J Cytol. 2012; 29: 200-202.
- Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek. 1998; 73: 331-371.
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nature Reviwews Neurology. 2017; 13: 13-24.
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect Dis. 2017; 17: 873-881.
- Leimann BC, Koifman RJ. Cryptococcal meningitis in Rio de Janeiro state, Brazil, 1994-2004. Cad Saude Publica. 2008; 24: 2582-2592.
- Philip KJ, Kaur R, Sangeetha M, Masih K, Singh N, Mani A. Disseminated cryptococcosis presenting with generalized lymphadenopathy. J Cytol. 2012; 29: 200-202.
- Suchitha S, Sheeladevi CS, Sunila R, Manjunath GV. Disseminated Cryptococcosis in an Immunocompetent Patient: A Case Report. Case Reports in Pathology. 2012.
- Azambuja AZ, Neto GW, Watte G, Antoniolli L, Goldani LZ. Cryptococcal Meningitis: A Retrospective Cohort of a Brazilian Reference Hospital in the Post-HAART Era of Universal Access. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018.
- Lui G, Lee N, Ip M, Choi KW, Tso YK, Lam E, Chau S, Lai R, Cockram CS. Cryptococcosis in apparently immunocompetent patients. QJM. 2006; 99: 143-151.
- Kashef HBH, Franco-Paredes C, McCollister B, Shapiro L, Beckham JD, Henao-Martínez AF. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Send to Mycoses. 2018; 61: 314-320.
- Chuang YM, Ho YC, Chang HT, Yu CJ, Yang PC, Hsueh PR. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis. 2008; 27: 307-310.
- Amaral DM, Rocha RC, Carneiro LE, Vasconcelos DM, Abreu MA. Disseminated cryptococcosis manifested as a single tumor in an immunocompetent patient, similar to the cutaneous primary forms. An Bras Dermatol. 2016; 91: 29-31.
- Mada P, Nowack B, Cady B, Joel Chandranesan AS. Disseminated cryptococcosis in an immunocompetent patient. BMJ Case Rep. 2017.
- Jain BB, Bose D, MondaL R, Chattopadhyay S. Disseminated cryptococcosis in an Immunocompetent Child. 2017; 33: 77-80.
- Ruan Q, Zhu Y Chen S, Zhu L, Zhang S, Zhang W. Disseminated cryptococcosis with recurrent multiple abscesses in an immunocompetent patient: A case report and literature review. BMC Infectious Diseases. 2017; 17: 369.
- Costa CR. et al. Infecções fúngicas em pacientes HIV positivos: Revisão da Literatura sobre Cryptococcosis e Histoplasmose. Estudos. 2014; 41: 843- 854.
- Pizani AT, Santos MO. Cryptococcosis em pacientes HIV positivos: revisão sistemática da literatura. Revista Saúde Uni Toledo. 2017; 1: 90-106.
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010; 50: 291-322.
- Yang Y, Shen Y, Zong W, Cui P. Disseminated cryptococcosis. Indian J Dermatol Venereol Leprol. 2016; 82: 206-208.