
Research Article
J Bacteriol Mycol. 2020; 7(1): 1122.
Antifungal Activity of Some Mixed Ligand Complexes Incorporating Schiff Bases
Miloud MM1, El-ajaily MM2*, Al-noor TH3 and Albarki NS4
¹Department of Botany, Faculty of Science, Benghazi University, Al-abyar branch, Libya
²Department of Chemistry, Faculty of Science, Benghazi University, Benghazi, Libya
³Department of Chemistry, Ibn -Al-Haithem College of Education for Pure Science, Baghdad University, Baghdad, Iraq
4Department of Chemistry, Faculty of Science, Ajdabyia University, Ajdabyia, Libya
*Corresponding author: El-ajaily MM, Department of Chemistry, Faculty of Science, Benghazi University, Benghazi, Libya
Received: January 31, 2020; Accepted: February 20, 2020; Published: February 27, 2020
Abstract
The Schiff base (HL1), namely; [(S,Z)-2-((2-hydroxy-1-phenylethylidene) amino)-3-(4-hydroxyphenyl)propanoic acid] was synthesized by the condensation of 2-hydroxyacetophenone and L-Tyrosine Whereas, the other Schiff base (HL2), namely; (E)-4-((2-(2,4-dinitrophenyl)hydrazono)methyl)-N,Ndimethylaniline] was synthesized by refluxing 4-dimethylaminobenzaldehyde and 2,4-dinitrophenylhydrazine. The first Schiff base (HL1) is used as primary ligand, whereas, the second Schiff base (HL2) is used as secondary ligand to prepare five mixed ligand complexes with Co(II), Ni(II), Cu(II), Zn(II) and Fe(III) ions. These synthesized mixed ligand complexes were characterized by using several spectroanalytical techniques. The data reveals octahedral geometry for all the mixed ligand complexes. In addition, these compounds were also tested for their antifungal activities against Aspergillus niger, Aspergillus flavus, Altarnaria alternata, Rhizopus stolonifer.
Keywords: Schiff Bases; Mixed Ligand Complexes; Antifungal Activity
Introduction
The Schiff base compounds are commonly used as ligands in the synthesis of coordination compounds [1,2]. These compounds have an important role due to their physiological and pharmaceutical activities [3,4]. Miloud et al [5] have studied the antifungal activities of five mixed ligand complexes with a Schiff base as main ligand and 2-aminobenzoic acid as co-ligand by using agar well diffusion method. It was found that some of the complexes are most active against Aspergillus niger, Aspergillus flavus, Altarnaria alternata, Rhizopus stolonifer.
Nair et al [6] have also reported four complexes with a Schiff base formed from 3-aminobenzoic acid and indole-3-carboxaldehyde. The antimicrobial activities of these compounds were examined by diffusion method and the results confirmed that the free Schiff base is less active than its metal complexes. Three complexes of divalent metal ions with a Schiff base formed from the reaction of 4-diethylaminosalicylaldehyde and 1-(4-aminophenyl) ethanone oxime have been investigated. The results are in good agreement with square planar geometry for Ni(II) and Cu(II) complexes, while, a tetrahedral geometry for Co(II) complex [7]. The biological activity of all the compounds was examined against some selected pathogenic organisms. Andiappan et al [8] have reported a series of lanthanide complexes with a Schiff base obtained by the condensation of 2,6-diaminopyridine and anthracene-9-carbaldehyde. The Schiff base and its metal complexes were tested for their cytotoxic activity against cervical (HeLa) and human breast cancer (MCF7) cell line.
In this study, we have reported the antifungal activities of some Schiff base mixed ligand complexes of Co(II), Ni(II), Cu(II), Zn(II) and Fe(III) chelates against some fungal strains such as A. niger, A. flavus, A. alternata, R. stolonifer.
Experimental
Chemicals and physical measurements
All the chemicals and reagents used in this study were of AR grade. The characterizations such as CHN analyses, molar conductivity, magnetic moment measurements and spectral analyses of these synthesized Schiff base ligands and their mixed ligand complexes has already been reported [9]. But here we are reporting the antifungal activities of these compounds.
Synthesis of Schiff bases and their mixed ligand complexes
The Schiff base (HL1), namely; [(S,Z)-2-((2-hydroxy-1- phenylethylidene)amino)-3-(4-hydroxyphenyl)propanoic acid] was synthesized by the condensation of 2-hydroxyacetophenone and L-Tyrosine (Figure 1). Whereas, the other Schiff base (HL2), namely; (E)-4-((2-(2,4-dinitrophenyl)hydrazono) methyl)-N,N-dimethylaniline] was synthesized by refluxing 4-dimethylaminobenzaldehyde and 2,4-dinitrophenylhydrazine (Figure 2,3). The Schiff bases and their mixed ligand complexes have been reported earlier [9].
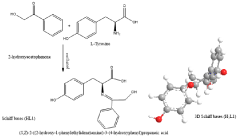
Figure 1: Structural of Schiff base (HL1).
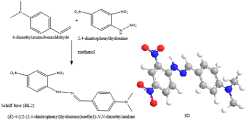
Figure 2: Schiff base (HL2).
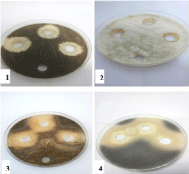
Figure 3: Structure of mixed ligand complexes.
Fungal species: Four test organisms such as A. niger, A. flavus, A. alternata and R. stolonifer were collected from the Laboratory of Applied Microbiology, University of Omar AL-Mukhtar, Libya. They were cultured in Petri plates containing Potato Dextrose Agar (PDA) media and incubated at 27°C for three days with periodic subculturing at 4°C.
Antifungal activity test: The antifungal activity of all these compounds was evaluated by agar well diffusion method [10]. All fungi were sub-cultured and prepared for the assessment of ligands and their complexes. The test compounds were dissolved in DMF solution. The PDA medium was poured into the sterile petri plates and allowed to solidify. The inoculum used was prepared using the fungal species from a 72-hour culture on PDA. The fungal suspension of each test fungi was evenly spread over the media by sterile cotton swabs. The plates have been kept to dry and a sterile cork borer (7mm in diameter) were then used to punch wells in the agar medium. Subsequently, wells were filled with 20μl of each compounds at various concentration of 25, 50 and 100 mg/mL and allowed to diffuse at room temperature for 15 min. The plates were incubated at 27°C for 48-72 hrs. The experiments were conducted in three duplicates. After the incubation, the plates were observed for formation of clear inhibition zone around the well indicated the presence of antifungal activity evaluated by measuring the diameter of the inhibition zone around the hole (mm).
Results and Discussion
In vitro antifungal activity
Our Schiff bases are tridentate (HL1) and bi-dentate (HL2) ligands forming very stable complexes with the metal ions. On complexation, the ligand with the O donor system might have inhibited enzyme production, since enzyme, which requires a free –OH group for their activity appear to be especially susceptible to deactivation by the ions of the complexes.
The in vitro antifungal activities in three concentrations for these compounds are shown in (Table 1) and (Figure 4-17). DMF is used as negative control. The complex, [Fe(L1)(L2)H2O].2H2O displayed significant antifungal activity compared to other complexes [11]. The salt, CoCl2.6H2O showed significant activity against A. alternata, moderate activity towards A.niger and R. stolonifer and less activity against A. flavus. NiCl2.6H2O exhibited high activity against A.niger and R. stolonifer, whereas, moderately effective against A. flavus and A. alternata. CuCl2.2H2O exhibited moderate activity against R. stolonifer and less activity against A.niger. But no activity shown against A. flavus and A. alternate. ZnCl2 exhibited high activity against A.niger, moderate activity against R.stolonifer, but no activity against A. flavus and A. alternate. FeCl3.6H2O showed moderate activity against A. flavus, R. stolonifer and A. alternata, but no activity against A.niger. The Schiff base ligand (HL1) revealed higher activity against A. alternata and moderate activity against other tested fungal strains. The Schiff base ligand (HL2) did not show any activity. From the above results it is clearly observed that the fungal activity depends upon the nature of metal ion [12]. The variation in the activity of different metal complexes against different microorganism depends on their impermeability of the cell. The lipid membrane surrounding the cell favors the passage of any lipid soluble materials and it knows that lipo solubility is an important factor controlling antifungal activity [13,14].
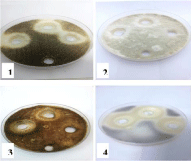
Figure 4: Effect of CoCl2.6H2O on 1- Aspergillus niger, 2- Aspergillus flavus,
3- Alternaria alternata and 4- Rhizopus stolonifer at 25,50 and 100 mg/ml
concentrations, respectively (from right to left) compared with control.
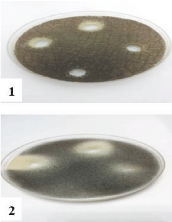
Figure 5: Effect of NiCl2.6H2O on 1- Aspergillus niger, 2- Aspergillus flavus,
3- Alternaria alternata and 4- Rhizopus stolonifer at 25,50 and 100 mg/ml
concentrations, respectively (from right to left) compared with control.
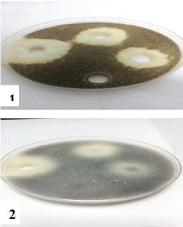
Figure 6: Effect of CuCl2.2H2O on 1- Aspergillus niger and 2- Rhizopus
stolonifer at 25,50 and 100 mg/ml concentrations, respectively (from right to
left) compared with control.
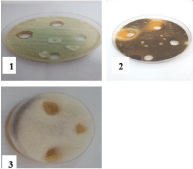
Figure 7: Effect of ZnCl2 on 1- Aspergillus niger and 2- Rhizopus stolonifer
at 25,50 and 100mg/ml concentrations, respectively (from right to left)
compared with control.
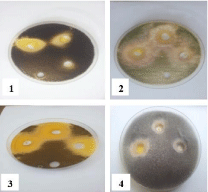
Figure 8: Effect of FeCl3.6H2O on 1- Aspergillus flavus, 2- Alternaria
alternate and 3- Rhizopus stolonifer at 25,50 and 100 mg/ml concentrations,
respectively (from right to left) compared with control.
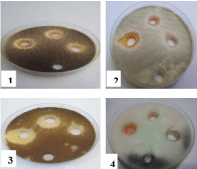
Figure 9: Effect of C18H21NO5(HL1) on 1- Aspergillus niger, 2- Aspergillus
flavus, 3- Alternaria alternata and 4- Rhizopus stolonifer at 25,50 and 100
mg/ml concentrations, respectively (from right to left) compared with control.
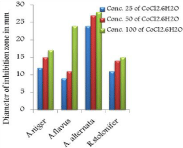
Figure 10: Effect of (Fe(L1)(L4)H2O).2H2O on 1- Aspergillus niger, 2-
Aspergillus flavus, 3- Alternaria alternata and 4- Rhizopus stolonifer at 25,
50 and 100 mg/ml concentration, respectively (from right to left) compared
with control.
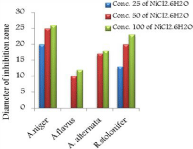
Figure 11: Effect of CoCl2.6H2O on different types of fungi.
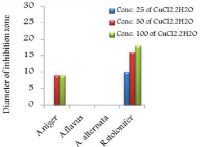
Figure 12: Effect of NiCl2.6H2O on different types of fungi.
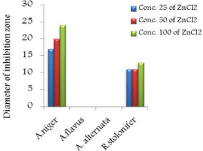
Figure 13: Effect of CuCl2.6H2O on two types of fungi.
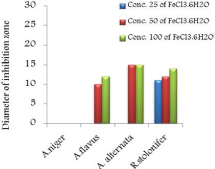
Figure 14: Effect of ZnCl2 on two types of fungi.
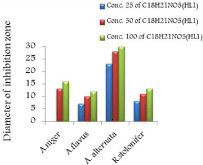
Figure 15: Effect of FeCl3.6H2O on three types of fungi.
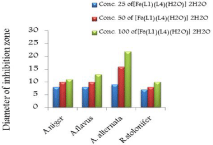
Figure 16: Effect of C18H21NO5(HL1) on different types of fungi.
TABLECREATED
Table 1: Antifungal activity for the metal salts, Schiff bases and mixed ligand complexes
Conclusion
The mixed ligand complexes with Schiff bases formed by the condensation of [2-hydroxyacetophenone and L-Tyrosine] (HL1) as primary ligand and [4-dimethylaminobenzaldehyde with 2,4-dinitrophenylhydrazine] as secondary ligand (HL2) are electrolytic in nature except Fe(III) mixed ligand complex. The complex, [Fe(L1)(L2)H2O].2H2O displayed significant antifungal activity compared to other tested metal complexes. The Schiff base ligand (HL1) revealed higher activity against A. alternata and moderate activity against other tested fungal strains. The Schiff base ligand (HL2) did not show any activity.
References
- Rehman W, Saman F, and Ahmed I. Synthesis, characterization and biological study of some biologically Potent Schiff base transition metal complexes, Russ. Coord. Chem.2008; 34: 678-682.
- MM El-ajaily, AK Sarangi, RK Mohapatra, SS Hassan, RN El-daghare, PK Mohapatra, et al. Transition metal complexes of (E)-2((2-hydroxybenzylidene) amino-3-mercaptopropanoic acid: XRD, Molecular modeling, Anticancer and Molecular docking Studies. ChemistrySelect. 2019; 4: 9999-10005.
- Wang M. and Wang LF. Antitumour Activity of transition metal complexes with the thiosemicarbazone derived from 3- acetylumbelliferone. Trans. Met. Chem. 2000; 26: 307-310.
- Mohapatra RK, Das PK, Pradhan MK, Maihub AA, and Elajaily MM. Biological aspects of Schiff base–metal complexes derived from benzaldehydes: an overview. Journal of the Iranian Chemical Society. 2018; 15: 2193-2227.
- Miloud MM, Alassbaly F, El-ajaily MM and Al-Noor TH. Antifungal Activities of Some Divalent and Trivalent Metal Complexes with 2-aminobenzoic acid and Schiff base derived from 4-dimethylaminobenzaldehyde and 2-aminophenol. J. Biol. Chem. Chron. 2016; 2: 42-46.
- Nair MS, Arish D and Joseyphus RS. Synthesis, characterization and Antifungal, Antibacterial and DNA cleavage studies of some heterocyclic Schiff base and its complexes. Journal of Soudi Chemical Society. 2012; 16: 83-88.
- Al-Zaidi BH, Hasson MM, Ismail AH. New complexes of chelating Schiff base: Synthesis, spectral investigation, antimicrobial and thermal behavior studies. Journal of Applied Pharmaceutical Science. 2019; 9: 45-57.
- Andiappan K, Sanmugam A, Deivanayagam E, Karuppasamy K, Kim HS and Vikraman D. In vitro cytotoxicity activity of novel Schiff base ligand–lanthanide complexes. Scientific Reports. 2018; 8: 1-12.
- Al-barki NS, Maihub AA, El-ajaily MM and Al-NOOR TH. Synthesis and physiochemical studies of some mixed Schiff bases complexes. Academic Journal of Chemistry. 2016; 1: 66-75.
- Mohapatra RK, Mishra UK, Mishra SK, Mahapatra A and Dash DC. Synthesis and characterization of transition metal complexes with benzimidazolyl-2- hydrazones of oanisaldehydeand furfural. Journal of the Korean Chem. Soc. 2011; 55: 926-931.
- Rahman A, Choudry MI and Thomsen WJ. Bioassay Techniques for Drug Development: Harwood Academic Publishers, Netherlands. 2001.
- Alassbally FS, El-ajaily MM, Ben-Gwierif SF and Maihub AA. Preparation, Spectroscopic investigation and biological activity of new mixed ligand complexes. Journal of Chemical Society of Pakistan. 2014; 36: 1034-1042.
- Indu S, Arun K. Synthesis, characterization and antifungal activity of Schiff base and its metal complexes. European Journal of Pharmaceutical and Medical Research. 2016; 3: 363-365.
- Anandakumaran JM, Sundararajan L, Jeyakumar T, Uddin MN. Transition metal complexes of 4-aminobenzenesulfonamide 1,3-benzodioxole-5- carbaldehyde: Synthesis, characterization and biological activities. American Chemical Science Journal. 2016; 11: 1-14.