
Review Article
J Bacteriol Mycol. 2020; 7(4): 1138.
Can the Novel Marker 'Lipopolysaccharide Binding Protein’ Benefit the Diagnosis of Ventilator-Associated Pneumonia in Geriatric Patients?
Ozguler M*
Department of Infectious Diseases and Clinical Microbiology, Elazig Educational and Research Hospital, Elazig, Turkey
*Corresponding author: Muge Ozguler, Department of Infectious Diseases and Clinical Microbiology, Elazig Educational and Research Hospital, Elazig, Turkey
Received: May 27, 2020; Accepted: June 23, 2020; Published: June 30, 2020
Abstract
Aim: Patients on mechanical ventilation have an increased risk of developing ventilator-associated pneumonia. Acinetobacter baumannii is one of the bacteria that cause problems in the intensive care units. Lipopolysaccharide is a potent toxin found in the cell walls of the gram-negative bacteria. Mean serum concentration of lipopolysaccharide binding protein is 5-20 mg/mL. Lipopolysaccharide binding protein levels of >200 mg/mL are noted in situations of acute phase response. We aimed to determine the diagnostic benefits of Lipopolysaccharide binding protein in older patients with A. baumannii-related ventilator-associated pneumonia in the intensive care units.
Materials and Method: In total, 100 patients were included in the study. Fifty patients with clinical and radiological findings, also with higher C-reactive protein levels and culture positivity for A. baumannii were included in the patient group and 50 patients with no clinical radiological findings and lower C-reactive protein levels, but culture positivity for A. baumannii were included in the colonisation (control) group, (mean age; 74 and 77 years, respectively). Procalcitonin and lipopolysaccharide binding protein levels were studied in each groups.
Result: The C-reactive protein, Procalcitonin and lipopolysaccharide binding protein levels were found significantly higher in the patient group than in the colonisation group.
Conclusion: Evaluation of clinical findings of the C-reactive protein, Procalcitonin and lipopolysaccharide binding protein levels in these patients may be helpful for differential diagnosis of colonisation and infection. We believe that lipopolysaccharide binding protein is a new marker that can contribute to VAP diagnosis in the geriatrics.
Keywords: Ventilator-Associated Pneumonia; Acinetobacter baumannii; Lipopolysaccharide Binding Protein; C-Reactive Protein; Procalcitonin
Introduction
Mechanical ventilation is a medical equipment that aids in patients’ continuous or intermittent breathing. Patients on mechanical ventilation have an increased risk of developing Ventilator-Associated Pneumonia (VAP). VAP is defined as a pneumonia that occurs after 48-72 h of endotracheal intubation, with the following criteria: new or progressive infiltration, findings of systemic infection (fever and elevated white blood cell counts) and changes in sputum characteristics and detection of a causal agent [1]. In 2012, the incidence of VAP was reported as 0.0-4.4 for each 1000 days of ventilation [2].
Acinetobacter baumannii is one of the bacteria that cause infectious complications in Intensive Care Units (ICUs). Acinetobacter baumannii is a gram-negative, aerobic, pleomorphic, non-motile, low pathogenic and opportunistic bacillus. It is commonly acquired from wet environments. Skin, respiratory and oropharyngeal colonisation is noted in individuals in ICUs [3,4]. Acinetobacters are important to be controlled as they cause progressive antimicrobial drug resistance and also make therapeutic management difficult [4].
Lipopolysaccharide (LPS) is a potent toxin found in the cell walls of gram-negative bacteria [5]. Even less than 1 pg/mL can cause macrophage activation. LBP is a triplet clone molecule that binds to Lipid A of LPS which is released from the surface of macrophages and monocytes. Thus, activation of phagocytosis, endocytosis and bacterial defences occurs [6].
LBP is a new marker for the determination of the severity of the infectious diseases and the response to the treatment. It is an acute phase reactant such as C-Reactive Protein (CRP) and Procalcitonin (PCT). Major amount of LBP is released from the liver and is also synthesised in the lungs [6,7]. Dramatic increase in the LBP levels is observed in inflammatory responses to infections such as sepsis [8].
LBP is a 65-kDa protein [9]. The mean serum concentration of LBP is 5-20 mg/mL. In case of an acute phase response, LBP levels of >200 mg/mL are observed [10].
Procalcitonin (PCT) is a peptide precursor of calcitonin. It contains 116 amino acids and is released from the parafollicular cells, thyroid tissue, lungs and the neuroendocrine cells of the intestines and is synthesised in response to bacterial proinflammatory stimuli. There is a positive correlation between PCT levels and the severity of infections [11].
This study aimed to determine the diagnostic benefits of LBP, a novel marker, in older patients with A. baumannii-associated VAP and to evaluate the correlation among the LBP, PCT and CRP levels.
Materials and Method
Ethical approval for the study was obtained from the local ethical committee. The study was prospectively conducted between May 2015 and February 2017. In total, 100 patients were included in the study. Informed consent was obtained from the relatives of all the patients. Fifty patients with clinical, laboratory and radiological findings, higher CRP levels and culture positivity for A. baumannii were included in the patient group, and 50 patients with no clinical, laboratory and radiological findings, lower CRP levels, but culture positivity for A. baumannii were included in the colonisation (control) group.
Exclusion criteria were determined as follows:
Previously initiated antibiotic therapy for A. baumanniiassociated infection, which was detected in patients transferred from another clinic to ICU
Patients in whom a different infection focus had been detected and is receiving antibiotic therapy, affecting A. baumannii
Patients with findings that met the criteria of Systemic Inflammatory Response Syndrome (SIRS) and sepsis (such as burn and multi-organ trauma), when admitted to the ICU
Patients who were treated in the ICU before being transferred to the service and then readmitted to the ICU
The patients who were newly admitted to the ICU and sought treatment for primary disease, with A. baumannii being detected in the Endotracheal Aspirate (ETA) cultures, were included in the study. Five millilitres blood was obtained from the patients who had A. baumannii in their ETA cultures to determine the PCT, CRP and LBP levels. Blood centrifugation was performed and serum samples were stored at −80°C until further use. Follow-ups and treatments were initiated according to the culture antibiogram for A. baumanniirelated pneumonia.
Demographic data were collected and recorded for the study. Primary diseases that caused admissions to the ICU and the physical examination findings were also recorded. Laboratory findings such as leukocyte counts, CRP, PCT and LBP levels, infiltrations on chest radiographs, microbiological results and the days of mechanical ventilation were added to the list.
The ETAs were collected and sent for microbiological determination. Microorganisms were defined using BioMeriux Vitek 2 Compact automated system (BioMerieux Marcy I’Etoile, France).
VAP was diagnosed on the basis of a combination of clinical, radiological and laboratory findings, in accordance with the criteria of the Centre for Disease Control (CDC) [12]. CRP levels were evaluated using the Immage 800 (Beckman Coulter) with Immunochemistry System), the PCT levels using the Mini Vidas PCT kits (B.R.A.H.M.S assay, bioMerieux, Marcy L’Etoile, France) and the LBP levels using the ELISA kits (Immulite DPC; Biermann, Bad Nauhe, Germany). The lower limit of the PCT test positivity was 0.05 ng mL−1, as determined by the manufacturer.
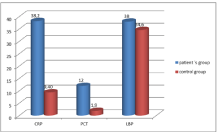
Graph: The CRP, PCT and LBP levels in patient and colonisation groups.
Statistical analysis
The data were analyzed by the SPSS 15. Normal distribution of the data was checked for each variable using the Kolmogorov- Smirnov test. The two-independent samples t-test (Mann–Whitney U) and Kruskal-Wallis tests were performed. The correlation of the mean CRP, PCT and LBP levels was studied using the Spearman’s correlation test. A p value of <0.05 was considered statistically significant.
Result
Data of 26 males and 24 females in the patient group and 34 males and 16 females in the control group were analysed in this study. The mean age of individuals in the patient group was 74 years and that in the control group was 77 years (min: 20 years, max: 95 years). The demographic data and mean CRP, PCT and LBP levels of the groups are presented in Table 1.
Variable
Value
Patient Control
P
Case n
50
50
-
Gender n, Male/Female
26/24
34/16
-
Mean Age
74
77
-
(95% CI: 65- 88)
(95% CI: 67- 86)
Mean WBC (mm3)
13500
12700
Mean CRP (mg/dl)
38.2
9.4
0.018*
(95% CI: 33.7- 42.7)
(95% CI: 5.6- 13.3)
Mean PCT (ng/ml)
12
1.9
0.006*
(95% CI: 6.9- 17)
(95% CI: 0.9-.2.8)
Mean LBP (μg/mL)
38
34.6
0.031*
(95% CI: 36- 39)
(95% CI: 30.6-38.7)
Table 1: Demographical characters of Patients and Control Groups.
We compared the CRP, PCT and LBP levels between the patient and colonisation groups. Additionally, the correlation between the groups was analysed. The CRP, PCT and LBP levels were significantly higher in the patient group than in the colonization group, with p values of 0.018, 0.006 and 0.031, respectively. Differences between the groups are presented in Graph.
Discussion
Multidrug Resistant (MDR) microorganisms-related VAP is the most important infectious complication in the ICUs. Patients in the risk group susceptible to MDR microorganisms are those who have been hospitalised for >2 days in the last 3 months, living in a nursing home, on haemodialysis and chemotherapy or on an antibiotic therapy in the last month. In the presence of the risk factors, infections caused by microorganisms such as Pseudomonas aeruginosa, Klebsiella, Enterobacter, Serratia, Acinetobacter, Stenotrophomonas maltophilia, Burkholderia cepacia and methicillin-resistant S. aureus should be considered [13].
One of the most important microorganisms that cause VAP is the A. baumannii. It is a multidrug resistant bacterium [4]. Microbiological colonisation occurs in patients in a few days in ICU. Distinctive diagnosis of colonisation and infection is a concern in these patients. When VAP is suspected in a patient in ICU, multiple tests are required for the differential diagnosis [14,15]. Therefore, a need to search new markers has emerged.
In this study, the mean CRP level (mg/dl) was higher in the patient group (38.2 mg/dl) than in the control group (9.4 mg/dl). Likewise, the mean PCT (ng/ml) and LBP (μg/mL) levels were higher in the patient group (12 ng/ml and 38 μg/mL, respectively) than in the control group (1.9 ng/ml and 34.6 μg/mL, respectively). The higher CRP, PCT and LBP levels were observed in geriatric patients with VAP than colonized geriatric patients and also higher correlation between LBP levels and CRP and PCT were observed in patients with VAP. The combination of CRP, PCT and LBP may be helpful for the differential diagnosis of colonisation and infection in A. baumanniirelated VAP.
CRP is an acute phase reactant. The CRP levels increase during inflammatory conditions such as rheumatoid pathologies, some cardiovascular diseases and infections. CRP levels increase from approximately 1 μg/mL to >500 μg/mL within 24-72 h of the onset of inflammation. However, CRP has some negative features such as late peak, suboptimal response and progressively remaining at the same levels [16,17].
As LBP is synthesised in the lung epithelial and alveolar cells, it can help to diagnose VAP; thus, it could be a new and important marker [9]. The combination of PCT and LBP can provide benefits in the diagnosis in patients with VAP. Previously, it was reported that combination of PCT and LBP increases the sensitivity from 88.5% to 96.3% and specificity from 53.2% to 66.7% [18].
Previously, LBP was studied in patients with sepsis and septic shock. It was reported that LBP levels have less diagnostic importance compared with the PCT and CRP levels, in situations of febrile neutropenia with sepsis [19]. In another study, LBP levels were studied in 54 neonatal patients with sepsis and significantly higher levels were found; significant cut-off level of LBP was 17.5μg/mL [20]. In another study, serum LBP levels were found significantly high in 58 cirrhotic liver patients with sepsis [21]. Similarly, significantly high LBP and PCT levels were found in 154 patients with severe sepsis [22]. In another study, LBP and PCT levels were studied in 100 patients with septic shock. However, no differences between the groups were found [23]. In our study, we observed lower LBP, PCT and CRP levels in the colonisation group. Our results are similar to those reported in previous studies in patients with sepsis [20-22]. However, we did not evaluate sepsis and septic shock status in our study.
In some other studies, even if higher LBP levels were noted in the patient group, statistical significance was not observed between the patient and control groups. Sakry et al. [24] found higher serum LBP levels patients with sepsis and septic shock in surgical ICU. However, no significant differences were found among the survivors and deceased.
Several diagnostic LBP levels have been previously reported in the literature. Carroll et al. [25] found higher mortality in patients with LBP values of >46 μg/mL. Prucha et al. [26] studied LBP levels in 68 patients in ICU, and the LBP levels were noted to be >29.8 μg/mL in patients with SIRS and sepsis. In this study, it was reported that LBP levels of >25μg/mL should be considered as a criterion for poor prognosis. In our study, we noted LBP levels in the patient and control groups as 38μg/mL and 34μg/mL, respectively. The difference in the levels among the studies might be attributable to the demographic and clinical factors and the reason of admission to ICU.
Additionally, PCT is a helpful diagnostic marker that increases in infection, such as LBP. In the present study, we observed mean PCT levels to be 12 ng/mL and 1.9 ng/mL in the patient and control groups, respectively, and a statistically significant difference was found (p < 0.05) between the groups. In previously reported studies, the cutoff levels for PCT have been presented as 1-5 ng/mL in infections [18,19]. Rumende et al. [18] studied PCT and LBP levels in VAP. The PCT levels of >5 ng/mL and LBP levels of >30μg/mL were found as criteria for poor prognosis in their study. In other previously reported studies, PCT was noted to be more sensitive than LBP on day 3 and day 7 examinations [27]. Higher PCT levels were found in a study which was conducted in 57 patients with community-associated pneumonia with mechanical ventilation and 61 patients with VAP in ICU [28]. Our results are similar to those of previously reported studies for Acinetobacter-related VAP infections.
In conclusion; The CRP, PCT and LBP levels were significantly higher in the patient group than in the colonisation group. Coevaluation of clinical findings such as CRP, PCT and LBP may help the clinician to differentiate between colonisation and infection. We believe that LBP is a new marker that can contribute to VAP diagnosis in geriatrics.
Acknowledgement
The authors would like to thank Enago (www.enago.com) for the English review.
References
- American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171: 388-416.
- Rosenthal VD, Al-Abdely HM, El-Kholy AA, Leblebicioglu H, Mehta Y et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010- 2015: Device-associated module. Am J Infect Control. 2016; 44: 1495-504.
- Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008; 47: 444-9.
- Manchanda V, Sanchaita S. Multidrug Resistant Acinetobacter. J Glob Infect Dis. 2010; 2: 291-304.
- Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis. Virulence. 2014; 5: 213-218.
- Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990; 249: 1429-31.
- Grube BJ, Cochane CG, Ye RD, Green CE, McPhail ME, et al. Lipopolysaccharide binding protein expression in primary human hepatocytes and HepG2 hepatoma cells. J Biol Chem. 1994; 269: 8477-8482.
- Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001; 98: 3800-8.
- Dentener MA, Vreugdenhil ACE, Hoet PHM, Vernooy JHJ, Nieman FHM, et al. Production of the acute-phase protein lipopolysaccharide binding protein by respiratory type II epitelial cells. Implications for local defense to bacterial endotoxins. Am J Respir Cell Mol Biol. 2000; 23: 146-1453.
- Gaı¨ni S, Koldkjaer OG, Pedersen C, Pedersen SS. Procalcitonin, lipopolysaccharide binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care. 2006; 10: R53.
- de Azevedo JR, Torres OJ, Beraldi RA, Ribas CA, Malafaia O. Prognostic evaluation of severe sepsis and septic shock: Procalcitonin clearance vs Delta Sequential Organ Failure Assessment. J Crit Care 2015; 30: 219; 9-12.
- Central of Disease Control (CDC). Pneumonia (Ventilator-associated (VAP) and non-ventilator-associated Pneumonia (PNEU)) Event.
- Hunter JD. Ventilator associated pneumonia. BMJ 2012, 344: e3325.
- Ntusi NBA, Badri M, Khalfey H, Whitelaw A, Oliver S, et al. ICU-Associated Acinetobacter baumannii Colonisation/ Infection in a High HIV-Prevalence Resource-Poor Setting. PLoS ONE. 2012: 7: e52452.
- del Mar Tomas M, Cartelle M, Pertega S, Beceiro A, Llinares P, et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk factors for colonisation and infection. Clin Microbiol Infect. 2005; 11: 540-546.
- Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004; 30: 261-77.
- Hisamuddin E, Hisam A, Wahid S, Raza G. Validity of C-reactive Protein (CRP) for diagnosis of neonatal sepsis. Pak J Med Sci. 2015; 31: 527-531.
- Rumende CM, Mahdi D. Role of combined procalcitonin and lipopolysaccharide-binding protein as prognostic markers of mortality in patients with ventilator-associated pneumonia. Acta Med Indones. 2013; 45: 89-93.
- Kitanovski L, Jazbec J, Hojker S, Derganc M. Diagnostic accuracy of lipopolysaccharide-binding protein for predicting bacteremia/clinical sepsis in children with febrile neutropenia: comparison with interleukin-6, procalcitonin, and C-reactive protein. Support Care Cancer. 2014; 22: 269-77.
- Leante-Castellanos JL, de Guadiana-Romualdo LG, Fuentes-Gutiérrez C, Hernando-Holgado A, García-González A, et al. The value of lipopolysaccharide binding protein for diagnosis of late-onset neonatal sepsis in very low birth weight infants. J Perinat Med. 2015; 43: 253-7.
- Chen YY, Lien JM, Peng YS, Chen YC, Tian YC, et al. Lipopolysaccharide binding protein in cirrhotic patients with severe sepsis. J Chin Med Assoc. 2014; 77: 68-74.
- Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999; 180: 1584-1591.
- García de Guadiana-Romualdo LM, Rebollo-Acebes S, Esteban-Torrella P, Jiménez-Sánchez R, Hernando-Holgado A, et al. Prognostic value of lipopolysaccharide binding protein and procalcitonin in patients with severe sepsis and septic shock admitted to intensive care. Med Intensiva. 2015; 39: 207-12.
- Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008; 3: 2014-22.
- Carroll SF, Dedrick RL, White M. Plasma levels of Lipopolysaccharide Binding Protein (LBP) correlate with outcome in sepsis and other patients (abstract101). Shock. 1997; 8: 101.
- Prucha M, Herold I, Zazula R, Dubska L, Dostal M, et al. Significance of lipopolysaccharide-binding protein (an acute phase protein) in monitoring critically ill patients. Critical Care. 2003; 7: 154-159.
- Samsudin I, Vasikaran SD. Clinical Utility and Measurement of Procalcitonin. Clin Biochem Rev. 2017; 38: 59-68.
- Park JH, Wee JH, Choi SP, Oh SH. The value of procalcitonin level in community-acquired pneumonia in the ED. Am J Emerg Med. 2012; 30: 1248-54.