
Review Article
J Bacteriol Mycol. 2020; 7(4): 1139.
Revisiting Host-Directed Adjunct Therapies in Tuberculosis
Fatima S1 and Dwivedi VP2*
1Department of Molecular Medicine, Jawaharlal Nehru University, New Delhi, India
2Department of Immunobiology Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, India
*Corresponding author: Dr Ved Prakash Dwivedi, Department of Immunobiology Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, India
Received: June 01, 2020; Accepted: June 29, 2020; Published: July 06, 2020
Abstract
Tuberculosis (TB) is a deadly disease caused by the intracellular obligate pathogen, Mycobacterium tuberculosis (M.tb) and affects one-third of the world’s population. Of all the infected individuals only around 5-10% of the individuals develop active TB disease but there is an emergence of approximately 10 million total TB cases with 1.7 million deaths annually, globally. It leads to maximum number of deaths due to an infectious agent. The treatment of TB by conventional drugs requires multiple antibiotics, is lengthy and therefore most of the time leads to non-compliance with the treatment. The problem of TB treatment has become even more challenging after the emergence of multidrugresistant and extremely drug-resistant TB. Therefore, strengthened efforts to find new TB drugs and immunotherapies which could improve the effects of the drugs and reduce the side effects associated with them, are urgently required. A Th1/Th2 imbalance in TB patients caused by decrease in host protective Th1 response and an increase in Th2 response plays a substantial role in the pathogenesis and progression of TB. Immunotherapies could modulate the immune system in patients with active TB disease or latent TB infection, helping in controlling the replication of M.tb better. Immunotherapy against TB infection principally aims to restore the Th1/Th2 balance by augmenting the necessary Th1 response and subduing the undue Th2 response. Therefore, the emerging field of immunotherapy needs to be further improved and studied to maximize its potential as combination therapy. This review discusses several potential immunotherapies with special emphasis on immuno-modulators. These immunotherapies can be used to intensify the potential of the standard anti-TB drugs while eliminating their side effects in the host, when they are administered as an adjunct with the conventional anti-TB drugs.
Keywords: Mycobacterium tuberculosis; Immunotherapy; Adjunct Therapy; MDR; XDR
Introduction
Tuberculosis (TB) remains a major public health concern. One fourth of the global population is estimated to have infected with M.tb. There are approximately 10 million new TB cases with 1.7 million deaths annually [1]. The only officially approved vaccine against M.tb is Mycobacterium bovis, Bacillus Calmette Güerin (BCG) that provides protection against miliary and childhood TB but proves to be unsuccessful in adults because of the absence of long lasting immunological memory. The only available treatment for TB is Directly Observed Treatment Short-course (DOTS) therapy [2]. Effective management of TB infection as well as the progression requires DOTS treatment, which is long, consists of several antibiotics and has multiple side-effects. These factors lead to noncompliance from the treatment, which ultimately results in the emergence of drug-resistant TB. It is noteworthy that drug-resistant TB is more challenging to treat and requires even a longer time [3]. This further increases the cost of TB control program in countries with a high percentage of infected population that have meager resources to invest in the control program. The World Health Organization (WHO) has reported a threefold increase in the incidences of Multiple Drug- Resistance (MDR) TB between 2013 and 2019. These M.tb strains are resistant to at least one of the antibiotics, isoniazid or revamping used in the treatment. XDR-TB (extensively drug resistant) is even more difficult to treat and the results of the treatment cannot be predicted. Therefore, the world is in extremely urgent need of developing new therapeutic strategies that has the potential of reducing the length of the Anti-Tuberculosis Therapy (ATT) and its associated side effects. Although, there are many new and efficient drugs in clinical trials but still the advancement in TB drug development is pretty slow and till date none of the drugs being tested so far has led to the shortening of the duration of the ATT [4]. Recent years have witnessed a huge interest in host directed therapies using immuno-modulators, which make the host immune system capable enough to fight against the bacteria on its own. This therapy mainly relies on maintaining a balance in the Th1/Th2 paradigm in the host organism by increasing the level of protective Th1 (T-helper 1) response and simultaneously reducing the level of Th2 response. It has also been established recently that Th17 host response also leads to protective immunity in TB disease [5]. Immunotherapies modulate the immune system in patients with both active and latent TB and thus assist in providing better control of M.tb replication [6]. The immuno-modulators are compounds derived naturally or synthesized and cytokines that have been reported to provide protection against TB by being used as an adjunct along with the DOTS therapy or even alone. These immuno-modulators target the biologically and clinically significant checkpoints in anti-mycobacterial directed pathways induced by the host [7]. They have also been described to reverse the side-effects of the DOTS therapy by providing lung protection and reducing the length of treatment of the DOTS therapy. Therefore, this hostdirected therapy using immuno-modulators is a promising approach which must be explored more elaborately for better control of the TB disease. This paper reviews the strategies and prospects for TB hostdirected therapy with the main focus on the immuno-modulators and their use in ATT as an adjunct therapy.
Host immune response to TB infection: The Th1/Th2 paradigm
Lungs are the primary organs, which gets infected by M.tb [8]. The encounter between M.tb and host immune response leads to the outcome of the TB disease- latent infection, active TB disease or complete eradication of the bacteria. T lymphocytes (CD4+) are the primary cell type that mediates the protective immune response to M.tb infection. This host immune response depends mainly on the cytokines secreted by the T helper (Th1) cells and Th17 cells, such as IFN-γ, IL-2, IL-12, IL-17 and IL-23. IFN-γ is the key factor in the containment of M.tb in the macrophages. These cytokines lead to the activation of macrophages. Activation of macrophages is important for the control of the disease by remarkably increasing the ability of macrophages to engulf and kill the pathogen by presenting the bacterial antigens to T-cells [9]. CD 8+ T-cells also produce similar cytokines as CD4+ T-cells and have cytolytic activity that can directly eliminate the pathogen. M.tb can evade the classical Th1-mediated response by inhibiting phagosomal maturation, lysosomal fusion, and MHC antigen presentation, resulting in its long-time survival in the host. At such a stage, CD8+ T-cells take the responsibility of getting rid of the bacteria by directly engulfing and killing the infected macrophages. Activated CD8+ T-cells can destroy the macrophages by releasing granzyme, which release intracellular bacteria and thus destroying the pathogen directly. Therefore, this dual strategy used by the host, which involves both macrophages and T-cells adequately, controls and eliminates M.tb infection. This mechanism of action is the immunological basis of TB treatment by Th1 cytokines [9]. Th2 cytokines, including IL-4, IL-10 and TGF-β, can impede the effect of Th1 cytokines. Thus, Th1 and Th2 cytokines interact and inhibit each other to maintain the T-cell homeostasis, killing the pathogen without significant damage to the host by a disproportionate and unbalanced immune response [10]. Remarkably, in TB patients, a Th1/Th2 imbalance with a decrease in Th1 cytokines and an increase in Th2 cytokines was observed, in the blood while Th1 cytokines were over-expressed in infected tissues [11]. This suggests that, the Th1/Th2 imbalance plays a significant role in the pathogenesis and expansion of TB disease, which could be explored for designing hostdirected immuno-therapeutics for an improved treatment of TB. The Th1/Th2 immune paradigm has been explained in Figure 1.
Tuberculosis: Pathogen burden and need for an alternative adjunct therapy
TB is a deadly disease causing approximately 1.7 million deaths each year [1]. The pathogenesis has become worse by the emergence of drug resistant strains. Treatment of drug-resistant TB is a major challenge in the prevention and eradication of TB. DOTS therapy is the only available treatment of the disease, which is characterized by long duration of treatment and administration of multiple drugs to achieve sterile cure of the infection and prevent occurrence of drug resistance [12]. The DOTS therapy characteristically is a 6 months treatment, comprising of an initial intensive period of 2 months followed by a 4 months continuation phase. During the first 2 months the patient is treated with four first line drugs namely, isoniazid (INH), rifampicin (Rif), ethambutol (ETH), and pyrazinamide. During the last 4 months the patient is given doses of Rif and INH. These drugs are accompanied by innumerable side effects, such as lung-toxicity, hepatitis, gastrointestinal problems, rashes, and renal failure [13]. These adverse effects coupled with the long duration of treatment often leads to non-compliance to the medications, and therefore increasing the risk of developing drug-resistant form of TB. The second-line of treatment of MDR patients, resistant to INH and Rif, consist of pyrazinamide, a fluoroquinolone along with an injectable antibiotic (amikacin or kanamycin), ethionamide, and cycloserine or para-aminosalicylic acid with the treatment, which lasts for 18-20 months [14]. Despite great efforts in finding new drugs for ATT, we still use the drugs which were discovered 40 years ago, except two new drugs, bedaquiline and delamanid that have been approved by FDA for treating MDR-TB patients [15-17]. We are in urgent need of novel drugs, which have enhanced efficacy as well as the ability to reduce the duration of the therapy while having an improved safety profile, compared to the currently available drugs [15]. With the decrease in the time taken for treatment, there would be a remarkable improvement and revolution in attaining cure of TB disease. This requires innovative efforts. Also, besides discovery of novel drugs, we need a therapy based on our understanding of the host-pathogen interactions. This would include not only the drugs that are bactericidal by themselves but also those which modulate the host immune pathways and clear the bacteria by a synergistic approach. This approach of eliminating the bacteria by enhancing the activity of the conventional drugs is known as adjunct therapy [18]. Adjunct therapies can be classified into host-directed or pathogendirected therapies based on their modulatory targets. Host-directed adjunct therapies are better to use, as they are not associated with emergence of antibiotic resistance. This dual approach of using standard drugs along with an adjunct therapy could enhance the effectiveness of ATT.
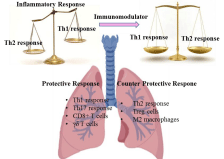
Figure 1: Immune response to TB in the lung of the infected individual:
Immune modulators maintain the homeostasis of Th1 and Th2 response in
the host which is disturbed by infection, thus protecting the host.
Therapy
Composition
No. of patients
Outcome
Refs
Cytokines and cytokine inhibitor
IL-2
50
In MDR-TB patients (led to better sputum conversion rates)
30
23
For treating MDR-TB patients (decrease AFB smear counts with daily IL-2 compared to control or pulse IL-2)
31
110
Newly infected TB patients (significant delays in culture conversion)
31
IFN-γ
5
For treating MDR-TB patients (all smear negative/improved)
25
7
For treating MDR-TB cases (led to no noticeable effect)
26
6
For treating MDR-TB cases (no noticeable effect)
26
Anti-TNF
16
For treating HIV-TB co-infected cases (better control of the disease)
39,40
Mycobacterium vaccae
Killed, intradermal
NA
Improved sputum conversion and X-ray changes
41
Capsule
41
Better sputum conversion rates
41
RUTI
Purified cellular fragments of M.tb
NA
In phase I and II clinical trials on LTBI cases or healthy volunteers leads to clearance of the pathogenic burden
42
Drugs/ compounds
High dose steroid
187
HIV- TB co-infected cases (led to rapid culture conversion after a month)
35
Levamisole
50
Newly diagnosed pulmonary TB patients (no effect on smear conversion but decrease in disease symptoms)
35
Albendazole
135
Pulmonary TB patients (not effective)
35
Thalidomide
15
Reasonable clinical improvement
40
30
No difference in clinical outcome of the disease
39
Autologous MSC
MSC
30
MDR or XDR patients showed improvement
51
Table 1: Summary of the Host-directed immunotherapeutics.
Host directed Immunotherapeutics in TB treatment
Immunotherapies are designed in such a way that they assist the host in controlling and eliminating the infectious agent by modulating the immune system. Whole M.tb, mycobacterial products, cytokines and novel drugs may possibly be utilized as immuno-modulators [19-22]. Host-directed immunotherapeutics that have been established for the treatment of TB in humans are summarized in Table 1. The immunotherapeutic strategy for TB therapy relies on reestablishing the Th1/Th2 balance by augmenting the Th1 response while suppressing the Th2 response simultaneously [23]. Therefore, host-directed immunotherapy for TB can be classified into three broad categories namely, immune-enhancing therapy with cytokines, immunosuppressive therapy, and immunomodulatory therapy.
Immune Enhancing Therapy with Cytokines
Interferon- γ therapy
Cytokines such as recombinant human γ-interferon (IFN-γ), recombinant human interleukin-2 (IL-2) and recombinant human interleukin-12 (IL-12) have been used in TB patients to enhance the protective Th1 host immune response. IFN-γ being the most prominent Th1 cytokine was found to enhance the capability of immune cells to engulf and significantly clear M.tb burden in murine model [24]. However, recent studies have random results after inhalation of IFN-γ during treatment of MDR-TB. Investigators have reported that inhalation of IFN-γ has variable efficacy during treatment of MDR-TB patients [25,26]. Administration of exogenous IFN-γ reduced the M.tb burden initially without completely curing the patient from the infection, even when a dose of aerosolized IFN-γ at 2 million units, three times a week was given for 6 months. It was observed that all patients reverted back or remained culture positive at the end of treatment [26]. Two randomized clinical trials investigating the potential of inhaled INF-γ as an additional therapy for MDR-TB and administration of INF-γ subcutaneously for the treatment against cavitary pulmonary TB were ended early owing to the lack of effectiveness of this therapy [25]. This therapy could not be implemented in TB treatment because of several factors which led to its failure like patient to patient variation, dosage, number of patients enrolled, difference between mouse and human immune systems and that the patients were not IFN-γ-deficient. The reason may be that the exogenous administration of IFN- γ did not further upregulate the Th1 cytokine response in subjects whose IFN-γ- induced gene has already been up regulated by M.tb challenge. It has also been reported that even after supplementation of inhaled IFN- γ in the patients, the expression level of inducible NO synthase (iNOS) in macrophages from TB patients who inhaled IFN-γ was same as untreated TB patients [27,28]. These outcomes rendered this therapy ineffective, as it may be useful in patients with IFN- γ deficiency but not otherwise. Few, studies however show some promising results in the elimination of the TB disease [29]. However, before we draw final conclusions, more placebo-controlled randomized studies should be undertaken with more number of subjects.
Interleukin -2
Several clinical studies in the past decades have used IL-2 to enhance the host- immune response against M.tb. However, the result of these trials have been quite varying. IL-2 is an important Th1 cytokine, which on one hand has a bacteriostatic response by inducing granuloma formation while on the other hand downregulates the host immune response by promoting the proliferation of T-regulatory cells (Tregs). One group of investigators has proved that IL-2 treatment can lead to the decrease in the number of Acid Fast Bacilli (AFB) in the sputum test of MDR-TB patients [30]. However, a different group of investigators reported a contrasting observation. They while working with drug-sensitive strain of M.tb identified that IL-2 treated group of patients seemed to have more AFB in the sputum than placebo and they took a longer treatment time. Moreover, they showed no improvement in their symptoms with the treatment [31]. On top of these results these investigators reported that administration of IL-2 could lead to the reduction in the secretion of IFN- γ, the key cytokine against M.tb, and thus further exaggerating the situation [31]. There have been no significant trial studies in the past 10 years for IL-2 as an adjunct therapy for TB mainly because of the variability in the outcome in different group of patients. The low success rate of this therapy could be attributed to induction of Tregs which made the antigen specific T-cells less responsive. This therapy could be reassessed as adjunct either by changing the route of administration or changing the dose of IL-2 being given to the TB patients.
Interleukin-12
IL-12 is essential for protective immunity against M.tb. In a study done by Greinert et al, treatment of a pulmonary TB patient who was unresponsive to the DOTS therapy gave effective results in clearing the pathogen. The patient was administered 300ng/ kg body weight of IL-12 subcutaneously for a duration of 3 months (twice weekly) [32]. Despite significant improvement in his condition initially, there was a relapse of the disease, which required additional 5 months to get completely cured of the disease. IL- 12 and IFN-γ form a feedback loop in the stimulation of the Th1-mediated immune response to TB [33]. Once there is a significantly high expression level of IL- 12, IFN-γ starts getting secreted. However, high dose of IL-12 has numerous side effects including liver damage that limit the therapeutic efficacy and clinical applications of IL-12 [34]. This is a very big reason of no clinical trial of IL-12 adjunct treatment in TB being reported. IL-12 stimulates the Th1 immune response. But exogenous administration of IL-12 failed to execute any therapeutic outcome because of already present IL-12 in the host. This has also been described as the “ceiling effect” by some researchers [35]. Furthermore, supplemental IL-12 may promote the progression to the Th2 immune response using feedback mechanism, which may increase the pathology even more.
Therefore, it has been observed that inducing Th1 immune response exogenously by the use of supplemental cytokines may be effective only in patients lacking these cytokines otherwise it is not clinically useful.
Immunosuppressive Therapy Using Cytokines
Tumor Necrosis Factor- α
The contribution of immunosuppressive therapy in TB is significant only for patients with a robust immune system, such as the patients affected from scrofula, caseous pneumonia, tuberculous meningitis, tuberculous pericarditis, tuberculous peritonitis to name a few and having a remarkably positive tuberculin skin test. These patients have a high level of Tumor Necrosis Factor Alpha (TNF-α). TNF-α is responsible for the development and maintenance of granuloma [36]. Thus, its role is of importance in treatment of patients with latent TB. In these patients, anti-TNF-α therapy acts on suppressing the immune system so that the bacteria do not enter the dormant state [37]. However, in cases of active TB infection anti- TNF-α therapy has proved to be fatal [38]. There are three widely used inhibitors of TNF-α- infliximab, adalimumab and etanercept that have been approved for treating patients with rheumatoid arthritis, Crohn’s disease and psoriatic arthritis [39]. Researchers have found these drugs lead to increase in relapse rate of TB in patients [40]. This reactivation of the disease could be because of the fact that inhibiting TNF- α results in improper formation and maintenance of the granuloma, thus releasing the M.tb and indirectly supporting its replication in the host. Furthermore, some TNF-α inhibitors interfere with innate immune response such as phagolysosomal fusion and apoptosis and adaptive immune responses such as IFN- γ secretion and increase the level of regulatory T-cells [40]. Inhibiting TNF-α can therefore aggravate the clinical symptoms of the disease in patients with progressive TB while reducing the establishment of granulomas. High level of TNF- α can lead to progression of HIV; Therefor anti- TNF-α therapy is used in patients with HIV-TB coinfection [40]. However, TNF- α inhibitors when used in any treatment increase the risk of reactivation of TB and are not advisable for a person who has been infected with TB earlier in life.
Mycobacteria or Mycobacterial Product Based Vaccines Used as Adjunct with the Standard Treatment
Investigators have been trying to discover a therapy using whole mycobacteria or the products derived from it for treatment along with the existing TB drugs. M. vaccae and other uncharacteristic mycobacteria such as M. indicus pranii and M.marinum also have shown promising results in murine models by acting as immunomodulators [41]. However, the role of this therapy is controversial with different group of researchers having different observations regarding it effectiveness.
Some mycobacteria based vaccines also seem to be quite effective as adjunct-therapy like RUTI and some DNA based vaccines. RUTI is a vaccine made up of purified cellular fragments of Mycobacterium tuberculosis (M.tb) bacilli cultured under stress and delivered in liposomes. RUTI is designed to stimulate latency antigens, which under normal conditions are hidden from the immune system [42]. This vaccine has been found to be quite successful in animal model for both prophylaxis and immunotherapy potential. So far, it has been shown in Phase I and II clinical trials involving healthy volunteers and cases with LTBI that this vaccine is safe and immunogenic [42]. It exerts its therapeutic effect by the induction and activation of a T helper-1 (Th1) response [43]. These vaccine based therapeutic strategies are more relevant in the treatment of drug resistant TB and HIV-TB co-infection.
Vaccines expressing important M.tb genes, such as Hsp65, ESAT- 6, and Ag85A, have shown promising results in M.tb -infected mice [44]. These vaccines are known as DNA vaccines and have resulted in up to three-log increase in M.tb clearance in infected mice [44]. These vaccines have shown quite remarkable results in case of MDR and XDR TB cases. It has been reported that a DNA vaccine expressing Hsp65 and IL-12 genes removed the symptoms of the disease in mice infected with MDR-XDR-TB. This vaccine has been tested in primates and has been known to provide a 40% improvement in survival of the animals [45]. The results of using mycobacterial product based vaccines as adjunct therapy have been quite encouraging. These vaccines are in clinical trials to be tested for use as adjuncts to conventional chemotherapy.
Vitamin D Therapy
Vitamin D has an essential role in defense against pathogenic infections by its ability to promote the activity of macrophages [46]. Vitamin D (1, 25-dihydroxyvitamin D) deficient people are high risk of getting infected with TB. During M.tb infection, calcidiol, the precursor of vitamin D, inhibits the replication of the bacteria by inducing innate immune responses. Calcidiol is then converted to its active state calcitriol by CYP27B1 enzyme [47]. Calcitriol acts by recruiting reactive oxygen intermediates, antimicrobial peptides, and inducing autophagy [47]. Although the exact mechanisms still needs to be determined, studies have emphasized that vitamin D supplementation effects the transcription of antimicrobial peptides such as DEFB4/HBD2 and cathelicidin (LL-37) [48]. Further, research suggests that Vitamin D leads to the reduction in bacterial load by stimulating the production of IL- 1β in infected cells [48]. Therefore, the vitamin D therapy needs to be further examined for / its use as adjunct therapy to TB.
Treatment with Mesenchymal Stem Cells
Mesenchymal Stem Cells (MSC) are pluripotent cell capable of differentiating into different cell types. MSCs in adults are present in different tissues and organs, bone marrow MSC being the most studied. These cells constitute 0.01% of the total cells present in the bone marrow [49]. These cells have been investigated for their immunomodulatory properties of inducting robust pathogen-specific immune responses. MSCs exert their immunomodulatory effects by interaction with the cells of the immune system most typically the T cells by cell-to-cell contact and release of relevant cytokines, such as Tumor Growth Factor (TGF)-β and prostaglandin E2 [50]. In a clinical setting 30 patients infected with MDR-TB were treated by autologous MSCs and they showed improvement both in terms of disease symptoms and time of treatment [51].
Immunomodulatory Therapy Using Natural Compounds
The balance of Th1/Th2 paradigm is very crucial for fighting against M.tb. Immunomodulators are very important agents that keep the balance of Th1/Th2 levels in the patients. Simply strengthening the Th1 response by using cytokines has shown to display many side effects possibly due to different feedback mechanisms. Therefore, researchers have shifted to therapeutic strategies, which could upregulate, the Th1 response while downregulating the Th2 immune response simultaneously. Maintaining the balance of this Th1/Th2 response using immunomodulators has shown some very effective results recently. Many researchers from different parts of the world have shown that using natural compounds derived from plants or phytochemicals and some drugs have efficient immunomodulatory effects that when used with the traditionally used DOTS therapy increase the efficacy of the treatment while reducing its duration. These immunomodulators have been quite effective in the treatment of drug-resistant strains of M.tb, which is good news.
Our lab has been working on many plant-derived compounds, which have proved to be successful in treatment of TB when administered as an adjunct therapy. We propose that a combination therapy of DOTS with an immunomodulator provides better protection from the disease and prevents reactivation of the disease [52-55]. These compounds which are used as immunomodulatory agents have no side- effects unlike other immunotherapies tested as adjunct treatment. They have been reported to reverse the toxicity induced by the antibiotics used in DOTS therapy. These beneficial outcomes of the immunomodulatory therapy as promisingly emerging alternative therapeutic intervention. Many plant-derived compounds such as allicin, curcumin, berginin and gingerol are being investigated for their immunomodulatory potentials [56]. Treatment using immunomodulators is by far the most promising adjunct therapy which is based on the principle that host susceptibility is responsible for mycobacterial infections not just the bacteria. Restoring the balance of Th1/Th2 cytokines after infection may provide protective immunity to the host. As the conventional ATT involves targeting the bacteria, it may lead to generation of drug resitance [56]. Therefore, immunotherapy, which is aimed at strengthening and restoring the host immune balance, makes the host capable of eradicating the bacteria by utilizing its own pathways. However, an immunomodulator cannot be used alone to get rid of TB. The conventional antibiotics are crucial for eradication of the bacteria. Therefore, using a combination therapy comprising of antibiotics and an immunomodulator could provide sterile clearance of the disease without causing drug resistance. This dual therapy also has the potential of reducing the risk of reinfection and reactivation.
Conclusion
Drug discovery approaches for developing a novel drug against M.tb mainly target the bacterial genes essential for its replication and survival. Host-directed therapies are novel therapeutics that target the host pathways and non-pathogenic proteins. These host directed therapies have the potential to bring about a paradigm shift in the existing TB treatment. These therapies can be used as adjunct treatments along with the existing drugs. M.tb has coexisted with humans since ages and this prominent interaction between the host and the bacteria paves way for a number of new drug targets to be used in adjunct therapy. Recent progress in vaccine development program and use of host directed therapies have shown remarkably positive results. Although, there are many challenges to the development and implementation of adjunct therapies but the results of the treatment in drug resistant patients provides promising perspectives to explore immunotherapy as a treatment option as a combination therapy. Several clinical trials are investigating the role of immunomodulators as adjunct therapy in treatment of TB. However, significant investments and research is still required to implement the use of adjunct therapy and other host immune system-based interventions in the future for better prevention and cure of tuberculosis.
References
- WHO Global Tuberculosis Report 2019.
- Shah NS, Wright A, Bai GH, Barrera L. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007; 13: 380-7.
- Cohen KA, Abeel T, McGuire AM, Desjardins CA, Munsamy V, Shea TP, et al. Evolution of extensively drug-resistant tuberculosis over four decades: Whole genome sequencing and dating analysis of Mycobacterium tuberculosis isolates from KwaZulu-Natal. PLoS Med. 2015; 12: e1001880.
- Davies PD. The role of DOTS in tuberculosis treatment and control. Am J Respir Med. 2003; 2: 203‐209.
- Shen H, Chen Z. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell Mol Immunol. 2018; 15: 216-225.
- Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001; 193:271-280.
- Hawn TR, Matheson AI, Maley SN, Vandal O. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol Rev. 2013; 77: 608‐627.
- Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol 2013; 31: 605-33.
- Raja A. Immunology of tuberculosis. Indian J Med Res. 2004; 120: 213‐232.
- Bozzano F, Marras F, De Maria A. Immunology of tuberculosis. Mediterr J Hematol Infect Dis. 2014; 6: e2014027.
- Reljic R, Paul M J, Arias MA. Cytokine therapy of tuberculosis at the crossroads. Expert Rev Resp Med. 2009; 3: 53-66.
- Awofeso N. Anti-tuberculosis medication side-effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Organ. 2008; 86: B‐D.
- Forget EJ and Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006; 5: 231-249
- Nahid P, Mase SR, Migliori GB. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline [published correction appears in Am J Respir Crit Care Med. 2020; 201: 500-501]. Am J Respir Crit Care Med. 2019; 200: e93‐e142.
- Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect. Dis. 2014; 14: 327-340.
- Cox E and Laessig K. FDA approval of bedaquiline–the benefit-risk balance for drug-resistant tuberculosis. N. Engl. J. Med. 2014; 371: 689-691.
- Rya NJ and Lo JH. Delamanid: first global approval. Drugs. 2014; 74: 1041-1045.
- Worthington RJ and Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013; 31: 177-184.
- Wang P, Wang L, Zhang W, Bai Y, Kang J, Hao Y, et al. Immunotherapeutic efficacy of recombinant Mycobacterium smegmatis expressing Ag85B-ESAT6 fusion protein against persistent tuberculosis infection in mice. Hum Vaccin Immunother. 2014; 10: 150-158.
- Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS One. 2011; 6: e23826.
- Yang S, Li F, Jia S, Zhang K. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell Physiol Biochem. 2015; 35: 1276-1288.
- Van Rhijn I, Moody DB. CD1 and mycobacterial lipids activate human T cells. Immunol Rev. 2015; 264: 138-153.
- Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015; 21: 401-406.
- Achkar JM, Casadevall A, Glatman-Freedman A. Immunological options for the treatment of tuberculosis: evaluation of novel therapeutic approaches. Expert Rev Anti Infect Ther. 2007; 5: 461-474.
- Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon gamma via aerosol. Lancet. 1997; 349: 1513-1515.
- Koh WJ, Kwon OJ, Suh GY. Six month therapy with aerosolized interferon for refractory multidrug-resistant pulmonary tuberculosis. J Korean Med Sci. 2004; 19: 167-171.
- Raju B, Hoshino Y, Kuwabara K. Aerosolized gamma interferon (IFN-gamma) Induces expression of the genes encoding the IFN- gamma-inducible 10-kilo-dalton protein but not inducible nitricoxide synthase in the lung during Tuberculosis. Infect Immun. 2004; 72: 1275-1283.
- Kuchtey J, Fulton SA, Reba SM,Harding CV, Boom WH. Interferon mediates partial control of early pulmonary Mycobacterium bovis bacillus Calmette–Guerin infection. Immunology. 2006; 118: 39-49.
- Kaufmann SH, Lange C, Rao M, et al. Progress in tuberculosis vaccine development and host-directed therapies--a state of the art review. Lancet Respir Med. 2014; 2: 301-320.
- Johnson JL, Ssekasanvu E, Okwera A. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003; 168: 185-191.
- Johnson BJ, Bekker LG, Rickman R. rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber Lung Dis. 1997; 78: 1950-203.
- Greinert U, Ernst M, Schlaak M, Entzian P. Interleukin-12 as successful adjuvant in tuberculosis treatment. Eur Respir J 2001; 17: 1049-51.
- Feng CG, Jankovic D, Kullberg M. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL- 12 production. J Immunol. 2005; 174: 4185-4192.
- Elas-Lpez AL, Marquina B, Gutirrez-Ortega A. Transgenic tomato expressing interleukin-12 has a therapeutic effect in a murine model of progressive pulmonary tuberculosis. Clin Exp Immunol. 2008; 154: 123-133.
- Abate G, Hoft DF. Immunotherapy for tuberculosis: future prospects. Immunotargets Ther. 2016; 5: 37‐45.
- Algood HM, Lin PL, Yankura D. TNF influences chemokine expression of macrophages in vitro and that of CD11b1 cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004; 172: 6846-6857.
- Skomsvoll JF, Wallenius M, Koksvik HS. Drug insight: anti-tumor necrosis factor therapy for inflammatory arthropathies during reproduction, pregnancy and lactation. Nat Clin Rheumatol. 2007; 3: 156-164.
- Miller EA, Ernst JD. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J Clin Invest. 2009; 119: 1079‐1082.
- Harris J, Keane J. How tumor necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol 2010; 161:1-9.
- Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004; 39: 1254-1255.
- Gupta A, Ahmad FJ, Ahmad F, et al. Efficacy of Mycobacterium indicus pranii immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung. PLoS One. 2012; 7: e39215.
- Cardona PJ. RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis. 2006; 86: 273-89.
- Guirado E, Gil O, Caceres N, Singh M, Vilaplana C, Cardona PJ. Induction of a specific strong polyantigenic cellular immune response after shortterm chemotherapy controls bacillary reactivation in murine and guinea pig experimental models of tuberculosis. Clin Vaccine Immunol. 2008: 15: 1229-37.
- Lowrie DB, Silva CL. Enhancement of immunocompetence in tuberculosis by DNA vaccination. Vaccine. 2000; 18: 1712-1716.
- Okada M, Kita Y, Nakajima T, Kanamaru N. Novel therapeutic vaccine: granulysin and new DNA vaccine against tuberculosis. Hum Vaccin. 2011; 7: 60-67.
- Cardona PJ. RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis. 2006; 86: 273-89.
- Guirado E, Gil O, Caceres N, Singh M, Vilaplana C, Cardona PJ. Induction of a specific strong polyantigenic cellular immune response after shortterm chemotherapy controls bacillary reactivation in murine and guinea pig experimental models of tuberculosis. Clin Vaccine Immunol. 2008; 15: 1229-37.
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007; 179: 2060-63.
- Skrahin A, Ahmed RK, Ferrara G, Rane L, Poiret T, Isaikina Y, et al. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med. 2014; 2: 108-122.
- Joshi L, Chelluri LK, Gaddam S. Mesenchymal Stromal cell therapy in MDR/XDR tuberculosis: a concise review. Arch Immunol Ther Exp (Warsz). 2015; 63: 427-433.
- Skrahin A, Ahmed RK, Ferrara G, et al. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med. 2014; 2: 108-122.
- Dwivedi VP, Bhattacharya D, Singh , Bhaskar A, Kumar S, Fatima S, et al. Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J Ethnopharmacol. 2019; 243: 111634.
- Tousif S, Singh DK, Mukherjee S, Ahmad S, Arya R, Nanda R, et al. Nanoparticle-Formulated Curcumin Prevents Posttherapeutic Disease Reactivation and Reinfection with Mycobacterium tuberculosis following Isoniazid Therapy. Front Immunol. 2017; 8: 739.
- Ahmad S, Bhattacharya D, Kar S, Ranganathan A, Van Kaer L, Das G. Curcumin Nanoparticles Enhance Mycobacterium bovis BCG Vaccine Efficacy by Modulating Host Immune Responses. Infect Immun. 2019; 87: e00291-19.
- Kumar S, Sharma C, Kaushik SR, Kulshreshtha A, Chaturvedi S, Nanda RK, et al. The phytochemical bergenin as an adjunct immunotherapy for tuberculosis in mice. J Biol Chem. 2019; 294: 8555‐8563.
- Sieniawska E, Maciejewska-Turska M, Świątek L, Xiao J. Plant-based Food Products for Antimycobacterial Therapy. eFood. 2020.