
Research Article
J Bacteriol Mycol. 2020; 7(5): 1141.
Investigating the Diversity of Spoilage and Food Intoxicating Bacteria from Chicken Meat of Biratnagar, Nepal
Mahato A1,2, Mahato S1,3*, Dhakal K3 and Dhakal A3
¹AASRA Research and Education Academy Counsel, Biratnagar-6, 56613, Nepal, India
²Department of Military and Veterans Affairs, New Jersey Veterans Memorial Home, NJ, USA
³Department of Microbiology, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Nepal, India
*Corresponding author: Sanjay Mahato, Department of Microbiology, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Keshaliya Road, Janpariya Tole, Biratnagar-06, Nepal, India
Received: June 05, 2020; Accepted: July 02, 2020; Published: July 09, 2020
Abstract
Background: Foodborne diseases are global human health problems, especially in developing countries where substandard hygiene of food like meat and unsafe water supplies prevail which are aggravated by multidrug resistance.
Objectives: The study was designed for investigating the diversity of microbial population like E. coli, Staphylococcus spp, Vibrio spp, Salmonella, Shigella and Pseudomonas from raw chicken meat and their drug resistance.
Results: The major bacterial pathogens isolated were Shigella spp (60%) followed by Salmonella typhi (53.3%), Pseudomonas aeruginosa (46.7%) and E. coli (46.7%), Staphylococcus aureus (40%), Staphylococcus epidermidis (33.3%) and Vibrio spp (13.3%) were isolated. With four antimicrobial drugs, 57.1% isolates of E. coli were sensitive to cefotaxime and levofloxacin while 65% resistant to amoxicillin. S. aureus isolates were 100% sensitive to cefotaxime and amoxicillin while 50% were sensitive to erythromycin. All isolates of Vibrio were found to be 100% sensitive to levofloxacin and amoxicillin. The isolates of Shigella were 37.5% resistant to cefotaxime and levofloxacin; while 12.5% resistant to amoxicillin. Salmonella typhi showed 33.3% resistant to cefotaxime and 22.2% resistant to levofloxacin and amoxicillin. Pseudomonas aeruginosa is 28.6% resistant to amoxicillin; while 14.3% resistant to levofloxacin and cefotaxime.
Conclusion: The meat in retail shops of Biratnagar is highly contaminated so this could bring foodborne infections in the city. In this light, it is recommended that microbial assessment of fresh meats and other meat products for human consumption should be performed and proper cleanliness should be adopted to reduce a possible hazard.
Keywords: Staphylococcus; Pseudomonas; Spoilage; Chicken; Multidrug-Resistant
Abbreviations
spp: Species; MDR: Multi-Drug Resistant; g: Gram; MHA: Muller- Hinton agar; NA: Nutrient Agar; XLDA: Xylose Lysine Deoxycholate Agar; TCBS: Thiosulfate-Citrate-Bile Salts-Sucrose; MSA: Mannitol Salt Agar; mL: Milliliter; CLSI: Clinical and Laboratory Standards Institute; CTX: Cefotaxime; LE: Levofloxacin; AMX: Amoxicillin; E: Erythromycin; S: Sensitive; I: Intermediate; R: Resistance
Introduction
Spoilage is the procedure by which food is deteriorated and becomes unacceptable for humans or its quality is reduced making it inappropriate for sale or consumption [1]. Chicken is one of the most consumed meats which contains enough nutrition needed to support the growth of microorganisms [2]. Nepal produces 16662 metric ton of chicken meat [3]. Since meat contains fat, protein, minerals, carbohydrate and water, the quality of meat and meat products degrade because of digestive enzymes, microbial spoilage and lipid oxidation [4, 5]. Fat oxidation, protein degradation and the loss of other biomolecules are the results of meat spoilage process.
The intestinal tract and the skin of the animal are the main sources of these microorganisms. Chicken meat can be contaminated at several points throughout the processing operations like stunning, bleeding, skinning, evisceration and carcass splitting [6]. Moreover, cutting of meat at retail outlets could result in greater microbial growth owing to a large amount of exposed surface area, more readily available water, nutrient and greater oxygen penetration which leads to spoilage of meat [7]. Microorganisms reach the meat via butcher’s hands, utensils, and tools, clothing and apron, water, etc. The number can be multiplied during cutting and distribution [4].
Spoilage bacteria include Bacillus spp, Shigella spp, Streptococcus pyogenes, Proteus, Leuconostoc, Lactobacillus spp, Pseudomonas, Micrococcus, Streptococcus, Lactobacillus, Salmonella, Escherichia, Clostridium and Bacillus [8, 9]. The most important pathogens associated with meat include Salmonella, Staphylococcus aureus, Escherichia coli, Clostridium perfringens, Campylobacter jejuni, Listeria monocytogenes, Yersinia spp, and Aeromonas hydrophila, Acrobacter, Mycobacterium, Vibrio spp, etc [10,11].
Staphylococcal food intoxication generally occurs within one to six hours after the ingestion of contaminated water or food and symptoms are nausea, vomiting, abdominal cramps and diarrhea [12]. Vibrio cholerae produces cholera enterotoxin and responsible for the life-threatening secretory diarrhea. It is strongly aerobic, growth being scanty and slow anaerobically [13]. Since poultry meat is usually not consumed raw, these outbreaks are caused by undercooking or cross-contamination of ready-to-eat products with microbial contaminants from the raw poultry or others introduced during preparation of the food [14].
Widely using antibiotic in the poultry as treatment prophylaxis or growth promoters in livestock lead to widely spread antibioticresistant pathogens that cause the problem in the humans [15]. The prevalence of Multi-Drug Resistant (MDR) foodborne pathogens is increased by consumption of contaminated food because they are responsible for more serious disease than susceptible bacteria [16].
The main aim of this study was to assess the microbial diversity of spoilage and food intoxicating bacteria of raw meat from outlets of Biratnagar and to understand its possible role in spoilage and foodborne illnesses.
Materials and Methods
Sample Collection
Fifteen samples of raw chicken meat (25 g) were collected aseptically in a sterile plastic container from different meat shops in Biratnagar from July 2017 to August 2017. After collection, the samples were transported to the laboratory for further processing i.e., Isolation, identification.
Sample Processing
Twenty-five gram of meat sample was weighed and grinded with the help of motor and pestle. The initial dilution was prepared by adding 25 g of the sample into 225 mL of dilution blank (10-1), thoroughly mixing, and thereby performing serial dilution up to 10-7.
Isolation and Identification of Bacteria
For isolation of E. coli and Pseudomonas spp., Eosin Methylene Blue (EMB) agar (HiMedia, Mumbai, India) plate and Muller-Hinton Agar (MHA) (HiMedia, Mumbai, India) plate were spread with 0.1 mL inoculum from several dilutions respectively and incubated at 37°C for 24 hr and consequently sub-cultured onto Nutrient Agar (NA) (HiMedia, Mumbai, India) plate to get pure culture for further identification. For Salmonella typhi and Shigella spp, bacterial suspension was spread into Xylose Lysine Deoxycholate Agar (XLDA) (HiMedia, Mumbai, India) and incubated at 37°C for 24 hr and consequently sub-cultured onto NA to get pure culture for further identification. Similarly, Thiosulfate-Citrate-Bile salts- Sucrose (TCBS) Agar plate (HiMedia, Mumbai, India) and Mannitol Salt Agar (MSA) plate (HiMedia, Mumbai, India) were used for cultivation of Vibrio spp and Staphylococcus spp respectively. After aerobic incubation at 37°C for 24 hr, consequently, the characteristic S. aureus and S. epidermidis colonies were yellow and cream color respectively. Selected colonies were sub-cultured onto NA plates to get pure culture. The isolation of the bacteria was done by using spread plate technique. 0.1 mL of bacterial suspension from 10-3 and 10-4 was taken.
Microscopic and Biochemical Identification of the Isolates
Characterization and identification of the colony isolates were achieved by initial morphological examination of the colonies in the plate (macroscopically) for colonial appearance, size, elevation, form, edge, consistency, color, odor, opacity, and pigmentation, and the results were recorded. Gram’s staining, capsule and spore staining were done to the selected colonies for preliminary identification of the bacteria. The bacterial isolates were identified by cultural, physiological, morphological and biochemical tests as per Bergey’s manual of determinative bacteriology [17]. Biochemical identification of the isolated bacteria was done by particular tests such as catalase, oxidase, TSI, Indole, Methyl red, Voges-Proskauer and citrate tests, carbohydrate fermentation tests, coagulase, O/F tests and urease test [18].
Antibiotic Sensitivity Testing for the Isolates
The identified isolates were submitted to antimicrobial susceptibility testing according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [19]. Antibiotic sensitivity testing by disc diffusion method was performed for the isolates using commercially available antibiotic discs (HiMedia, Mumbai, India) on Muller-Hinton agar (MHA) (HiMedia, Mumbai, India). The organism was diluted to obtain a turbidity equivalent to the 0.5 McFarland test standard. Immediately a sterile cotton swab was dipped into bacterial suspension and lawn culture was performed on the surface of MHA plate. The antibiotic discs like Cefotaxime (CTX, 30 μg), Levofloxacin (LE, 5 μg), Amoxicillin (AMX, 10 μg), and Erythromycin (E, 15 μg) were placed on the plates. Then the plates were incubated at 37°C for 18 hours. After incubation, the zone diameter was measured and compared to the standard chart. Thereby the zone of inhibition was interpreted as Sensitive (S), Intermediate (I) or Resistance (R).
Result
A total number of 15 chicken meat samples were examined for the presence of bacteria. E. coli isolates appeared as greenish metallic sheen (Figure 1) on EMB agar plate. The large yellow colored colony on TCBS (Figure 2) represents Vibrio spp. Staphylococcus aureus and Staphylococcus epidermidis showed golden yellow and cream-colored colony respectively (Figure 3) on MSA agar plate and showed gelatin hydrolyzing activity (Figure 4). The bacterial pathogen isolated from chicken meat sample was Shigella (n=9, 60%) (Figure 5) followed by Salmonella (n=8, 53.3%), Pseudomonas (n=7, 46.7%) (Figure 6 and Table 1), E. coli (n=7, 46.7%); S. aureus (n=6, 40%); S. epidermidis (n=5, 33.3%) and V. cholera (n=2, 13.3%) and were identified by cultural and biochemical properties.
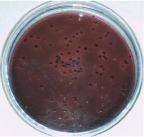
Figure 1: Growth of E. coli on EMB agar plate.
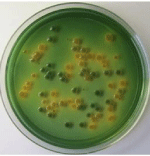
Figure 2: Growth of Vibrio spp on TCBS agar plate.
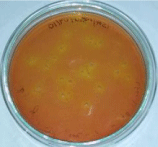
Figure 3: Growth of Staphylococcus spp on MSA agar plate.
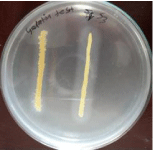
Figure 4: Gelatin test of S. aureus.
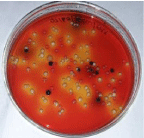
Figure 5: Growth of Salmonella and Shigella on XLD agar.
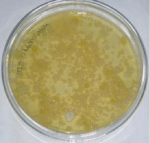
Figure 6: Growth of Pseudomonas on MHA.
Sample code
Microorganisms isolated
S1
Pseudomonas aeruginosa, S. aureus
S2
Salmonella typhi, Shigella, E. coli
S3
Shigella, Pseudomonas aeruginosa, Vibrio spp, S. aureus
S4
Shigella, Pseudomonas aeruginosa, E. coli, S. aureus
S5
Shigella, E. coli
S6
Pseudomonas aeruginosa, Salmonella typhi, Shigella, S. epidermidis
S7
Salmonella typhi, Shigella, S. aureus
S8
Shigella, Vibrio spp, S. epidermidis
S9
Pseudomonas aeruginosa, Salmonella typhi, E. coli
S10
Pseudomonas aeruginosa, E. coli
S11
Shigella, Salmonella typhi, S. aureus
S12
Salmonella typhi, E. coli, S. epidermidis
S13
Shigella, S. epidermidis
S14
Salmonella typhi, Pseudomonas aeruginosa, S. aureus, E. coli
S15
Salmonella typhi, S. epidermidis
Table 1: Types of microorganisms isolated in each sample.
While 100% of E. coli was found to be resistant to amoxicillin, 42.8% were resistant to cefotaxime and levofloxacin. All the isolates of S. aureus were sensitive to cefotaxime, levofloxacin, and amoxicillin, but 50% were resistant to erythromycin. All isolates of Vibrio spp were found to be sensitive to levofloxacin and amoxicillin. Of all Salmonella spp, 33.3% was found to be resistant to cefotaxime, while 22.2% to amoxicillin and levofloxacin. 37.5% of Shigella were resistant to cefotaxime and levofloxacin, and 12.5% resistant to amoxicillin. 14.3% Pseudomonas spp were resistant to cefotaxime and levofloxacin, and 28.6% to amoxicillin.
Discussion
A significant portion of meat and meat products are spoiled every year. Kantor et al [20] reported that approximately 3.5 billion kg of poultry and meat were wasted at the consumer, retailer and foodservice levels which have a substantial economic and environmental impact. The predominant bacterial pathogen isolated from chicken meat sample was Salmonella, Shigella, Pseudomonas, E. coli, S. aureus, S. epidermidis, and V. cholera. Different technical operations like stunning, bleeding, skinning, evisceration and carcass splitting involved in slaughtering may contaminate the meat. The intestinal tract and the skin of the animal are the main sources of these microorganisms. The composition of microflora in meat depends on several factors like pre-slaughter husbandry practices, age of the animal, handling during slaughtering, evisceration and processing, temperature controls during slaughtering, processing and distribution, preservation methods, type of packaging, handling and storage by consumer, the acidity of the meat, and the structure of the muscular tissue [21]. Microorganisms reach the carcasses via. butcher’s hands, tools, clothing, water, etc.
The reported 53.3% of Salmonella was higher than the findings (33.3%) of Balakrishnan et al [22] from Tamilnadu, India. Salmonellosis, E. coli enteritis and food poisoning by Staphylococcus are the major problems encountered by the consumers eating contaminated meat. Lower prevalence of Salmonella was reported by El-Aziz et al [23] at 44% in Egypt and Alali et al [24] at 31.5% in broiler chicken meat in Russia Federation. The high prevalence of Salmonella spp isolated from broiler meat in the present study indicates that poultry meat is one important source of human infection by foodborne poisoning salmonellosis. Salmonella infection in retail meat markets that consider risk of human infection has been through the handling of raw poultry carcass and products, handling in households, together with the consumption of undercooked meat [25].
The prevalence of Pseudomonas (46.7%) and Shigella (60%) was much higher than Bharatpur, Nepal as reported 1.5% and 3.9% respectively [26]. The raw chicken is stored at low temperature which reduces the rate of bacterial growth. Cold loving microbes such as Pseudomonas spp cause spoilage, making the meat smelly and slimy. Pseudomonas spp. are present everywhere and sources include drinking water, animals, human, plants, and from a variety of foods [14]. Shigella have highly evolved invasive systems which enable the bacteria to invade and multiply within the human intestinal epithelia, eventually leading to severe inflammatory colitis called bacillary dysentery or shigellosis [27].
The prevalence of E. coli (46.7%) was similar (47.2%) to the study of Adeyanju and Ishola [28]. This study showed a higher prevalence than that reported in Egypt where the rate was evaluated to 11.7% [29]. Escherichia coli, particularly psychrotropic enteric pathogenic strains E. coli 0157:H7, can grow on minimally processed vegetables and processed meat products at 4 -12°C causing hemorrhagic colitis. Enteroinvasive and enterotoxigenic types of E. coli can be a leading cause of foodborne diarrhea [4]. Escherichia coli is responsible for 25% of the infant diarrhea in developing countries [30].
The prevalence of Staphylococcus spp (73.3%) was slightly higher than the findings of Iran [31]. This study showed a higher prevalence of Staphylococcus spp than that of obtained by Gonçalves-Tenório et al. [32] which were reported as 38.5%. Staphylococcus aureus can be carried on human hands, in nasal passages, or throats. The bacteria are found in foods made by hand and, then, improperly refrigerated. Staphylococcus aureus is a leading cause of gastroenteritis resulting from the consumption of contaminated food [33]. The prevalence of V. cholera (13.3%) was adversely higher than findings of Sudan [31]. Poultry and poultry products are considered the major infectious routes for humans because different species of pathogenic and nonpathogenic microorganisms have been reported in poultry.
All of E. coli were resistant to amoxicillin which was greater than that stated as 65% resistant by Roth et al. [34]. The resistance of cefotaxime and levofloxacin in 42.8% E. coli was greater than the report of Roth et al. [34] as 23% to cefotaxime and 44% to levofloxacin. The total resistance of S. aureus to erythromycin was in agreement with Mwambete and Stephen [35]. The resistance to amoxicillin by Salmonella spp (22.2%) in this study was higher than Nepal [36] which stated to be 16.6%. This study showed 33.3% were resistant to cefotaxime and 22.2% to levofloxacin. Almashhadany [37] showed 100% sensitivity towards cefotaxime, while 100% were resistant levofloxacin. The resistance shown by Vibrio in this study towards erythromycin was similar to Akond et al. [38]. According to Elghazaly et al. [39], Pseudomonas showed high resistance (94.6%) to levofloxacin against this study (14.3%). Due to rapidly increasing in human population and changing in urbanization food habits, increasing in animal products consumption such as meats and the frequent and unnecessary use of antimicrobial agents for farming and therapeutic purpose in animals and human are bestowing to create resistant strains.
Conclusion
The marked growth of bacteria concludes that retail broiler meat is not suitable for consumption because of the poor condition of the market and the compromised hygienic practice employed by meat sellers and butchers. The presence of Salmonella, Staphylococcus, and Pseudomonas gives a warning signal for the possible occurrence of foodborne intoxication. Microbial assessment of fresh meats and other meat products processed and packaged for human consumption is, hence, emphasized and recommended to reduce a possible hazard. The contamination of poultry and poultry products should be prevented during handling, slaughter, and processing to protect the public from infections and diseases. The development of drug resistance to commonly used antibiotics by these common pathogens is a more serious matter of concern for food safety and public health.
Acknowledgement
We thankfully appreciate the efforts and inputs of AASRA Research and Education Academy Counsel for making this study possible and in preparation of the manuscript.
Conflict of Interest
Authors have no conflict of interest.
References
- Jay JM, Loessner MJ, Golden, DA. Modern Food Microbiology, 7th Edn, Springer Science and Business Media, NY. 2005; 63-101.
- Koffi-Nevry R, Koussemon M, Coulibaly SO. Bacteriological Quality of Beef Offered for Retail Sale in Cote d’ivoire. Am. J. Food Technol. 2011; 6: 835-842.
- Bhandari N, Nepali DB, Paudyal S. Assessment of bacterial load in broiler chicken meat from the retail meat shops in Chitwan. Nepal Int. J. Infect. Microbiol. 2013; 2: 99-104.
- Mahato S. Relationship of Sanitation Parameters with Microbial Diversity and Load in Raw Meat from the Outlets of the Metropolitan City Biratnagar, Nepal. Int. J. Microbiol. 2019; 2019: 3547072.
- Amit SK, Uddin MM, Rahman R, Islam SMR, Khan SM. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017; 6: 51.
- Addis M. Major Causes of Meat Spoilage and Preservation Techniques: A Review. Food Science and Quality Management. 2015; 41.
- Sharma KP, Chattopadhyay UK. Assessment of Microbial load of raw meat Samples sold in the Open Markets of city of Kolkata. IOSR-JAVS. 2015; 8: 24-27.
- Liu Q, Meng X, Li Y, Zhao CN, Tang GY, Li HB. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017; 18: 1283.
- Yusuf MA, Hamid TA, Hussain I. Isolation and Identification of Bacteria Associated with Balangu (Roasted Meat Product) Sold in Bauchi Nigeria. IOSR J. Pharm. 2012; 2: 38-48.
- Saroj SD, Maudsdotter L, Tavares R, Jonsson AB. Lactobacilli Interfere with Streptococcus pyogenes Hemolytic Activity and Adherence to Host Epithelial Cells. Front. Microbiol. 2016; 7: 1176.
- Jara S, Sánchez M, Vera R, Cofré J, Castro E. The inhibitory activity of Lactobacillus spp isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin. Anaerobe. 2011; 17: 474-7.
- Fletcher S, Boonwaat L, Moore T, Chavada R, Conaty S. Investigating an outbreak of staphylococcal food poisoning among travelers across two Australian states. Western Pac Surveill Response J. 2015; 6: 17-21.
- Tejan N, Datta P, Gupta V. Bacterial diarrhoea: a comprehensive review. Int. J. Pharm. Sci. Res. 2018; 9: 5015-31.
- Rouger A, Tresse O, Zagorec M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms. 2017; 5: 50.
- Hughes P, Heritage J. Antibiotic growth-promoters in food animals. FAO Animal Production and Health Paper, 2004; 129-152.
- Nair D, Kollanoor Johny A. Characterizing the Antimicrobial Function of a Dairy-Originated Probiotic, Propionibacterium freudenreichii, Against Multidrug-Resistant Salmonella enterica Serovar Heidelberg in Turkey Poults. Front. Microbiol. 2018; 9: 1475.
- Goodfellow M, K¨ampfer P, Busse HJ, Trujillo ME, Suzuki K, Ludwig W, et al. Bergey’s Manual of Systematic Bacteriology, Vol 5, 2nd edition, Springer- Verlag, New York, NY, USA. 2012.
- Cheesbrough M. Biochemical tests to identify bacteria. in District Laboratory Practice in Tropical Countries, Part II, 2nd edition, Cambridge University Press, New York, NY, USA. 2009.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 26th informational supplement. Wayne: CLSI. 2016.
- Kantor LS, Lipton K, Manchester A, Oliveira V. Estimating and addressing America’s food losses Food Rev. 1997; 20: 3-11.
- Cerveny J, Meyer JD, Hall PA. Microbiological Spoilage of Meat and Poultry Products. Sperber WH, Doyle MP, editors. In: Compendium of The Microbiological Spoilage, of Foods and Beverages Food Microbiology and Food Safety. Springer Science and Business Media, NY, 2009; 69-868.
- Balakrishnan S, Sangeetha A, Dhanalakshmi M. Prevalence of Salmonella in chicken meat and its slaughtering place from local markets in Orathanadu, Thanjavur district, Tamil Nadu. J Entomol Zool Stud. 2018; 6: 2468-2471.
- El-Aziz DM. Detection of Salmonella typhimurium in retail chicken meat and chicken giblets. Asian Pac. J. Trop. Biomed. 2013; 3: 678-681.
- Alali WQ, Gaydashov R, Petrova E, Panin A, Tugarinov O, Kulikovskii A, et al. Prevalence of Salmonella on retail chicken meat in Russian Federation. J. Food Sci. 2012; 75: 1469-1473.
- Saikia P, Joshi SR. Retail market poultry meats of North-East India – a microbiological survey for pathogenic contaminants. Res J Microbiol. 2010; 5: 36-43.
- Shrestha A, Bajracharya AM, Subedi H, Turha RS, Kafle S, Sharma S, et al. Multi-drug resistance and extended spectrum beta lactamase producing Gram negative bacteria from chicken meat in Bharatpur Metropolitan, Nepal. BMC Res. Notes. 2017; 10: 574.
- Shi R, Yang X, Chen L, Chang HT, Liu HY, Zhao J, et al. Pathogenicity of Shigella in chickens. PLoS One. 2014; 9: e100264.
- Adeyanju GT, Ishola O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria. SpringerPlus. 2014; 3: 139.
- Moawad AA, Hotzel H, Awad O, Tomaso H, Neubauer H, Hafez HM, et al. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut pathog. 2017; 9: 57.
- Shrivastava AK, Kumar S, Mohakud NK, Suar M, Sahu PS. Multiple etiologies of infectious diarrhea and concurrent infections in a pediatric outpatientbased screening study in Odisha, India. Gut pathog. 2017; 9: 16.
- Javadi A, Safarmashaei S. Study of Enterobacteriaceae Contamination Level in Premises of Poultry Slaughterhouse with HACCP System. J Anim Vet Adv. 2011; 10: 2163-2166.
- Gonçalves-Tenório A, Silva BN, Rodrigues V, Cadavez V, Gonzales-Barron U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods. 2018; 7: 69.
- Das P, Mazumder PB. Prevalence of Staphylococcus in raw meat samples in Southern Assam, India. IOSR-JAVS. 2016; 9: 23-29.
- Roth N, Käsbohrer A, Mayrhofer S, Zitz U, Hofacre C, Domig KJ. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview Poult. Sci. 2019; 98: 1791- 1804.
- Mwambete KD, Stephen WS. Antimicrobial resistance profiles of bacteria isolated from chicken droppings in Dar Es Salaam. Int. j. pharm. pharm. sci. 2015; 7: 268-271.
- Saud B, Paudel G, Khichaju S, Bajracharya D, Dhungana G, Awasthi MS, et al. Multidrug-Resistant Bacteria from Raw Meat of Buffalo and Chicken, Nepal. Vet. Med. Int. 2019; 2019: 7960268.
- Almashhadany DA. Occurrence and antimicrobial susceptibility of Salmonella isolates from grilled chicken meat sold at retail outlets in Erbil City, Kurdistan region, Iraq. Ital. J. Food Saf. 2019; 8: 8233.
- Akond MA, Alam S, Hasan SMR, Uddin SN, Shirin M. Antibiotic Resistance of Vibrio cholerae from Poultry Sources of Dhaka, Bangladesh. Adv Biol Res. 2008; 2: 60-67.
- Elghazaly EMM, Sedeek EK, Khalil SA. Molecular Characterization of Some Bacteria Isolated from Broiler Chickens Showing Respiratory Manifestations. Alex. J. Vet. Sci. 2017; 55: 98-106.