
Case Report
J Bacteriol Mycol. 2021; 8(4): 1175.
Postnattaly Acquired Cytomégalovirus Infection in Term Infant: A Case Report
Faverge B*, Attou D, Brunod IU, Caherec C, Caillot M, Dookna P, Forler J, Marrec C and Ticus I
Service De Pédiatrie-Néonatologie, Centre Hospitalier Martigues-Les-Rayettes, Boulevard Des Rayettes, France
*Corresponding author: Bernard Faverge, Service De Pédiatrie-Néonatologie, Centre Hospitalier Martigues- Les-Rayettes, Boulevard Des Rayettes, BP 50248, 13698 Martignes Cedex, France
Received: May 11, 2021; Accepted: June 12, 2021; Published: June 19, 2021
Abstract
Regarding a case of postnatal Cytomegalovirus (CMV) infection, the authors report that this infection transmitted through breast milk can be severe even in term newborns and that it requires specific antiviral therapy measures.
Introduction
The Cytomegalovirus (CMV) or herpes virus 5 belongs to the family of herpes viridae. It is a virus. It is a DNA virus of which humans are the only reservoir. The virus can persist in CD34+ precursors for life.
Immunity is acquired but incompletely protective, resulting in reactivations. The seroprevalence of pregnant women is around 85%. Fetal transmission is asymptomatic in 90% of cases at birth, hence the need for routine hearing screening in newborns. When symptomatic, it is a source of intrauterine growth restriction, neurosensory damage, microcephaly, ocular involvement (chorioretinitis), pneumonia, hepatitis, purpura [1].
Postnatal CMV can be transmitted through breast milk with hepatitis, marrow suppression, pneumonitis, Note that it can also be transmitted by blood transfusion, which is avoided by the use of negative CMV-IgG blood.
We report the case of a 4.5 month old infant who was hospitalized with a clinical picture of trailing bronchiolitis, since it had been progressing for about 3 weeks.
Case Presentation
History
Full term newborn, weight 3 kg 570, normal neonatal period. Exclusive breastfeeding.
At 2 months, the mother presented with a Staphylococcus Aureus breast abscess requiring a surgical approach. The mother continues to breastfeed with the unaffected breast; but two months later a second curettage-drainage operation will be necessary with complete cessation of breastfeeding at 4 months.
Clinical
Onset of signs with cough.
Paleness, general condition impairment with asthenia, hypotonia.
Polypnea. Oxygen dependence.
Standard biology
CBC: anemia, no lymphopenia, monocytosis, thrombocytopenia.
Normal bilirubinaemia, normal transaminitis (ALT, AST).
Thrombocytopenia and mononucleosis syndrome lead to CMV.
Virology: Maternal CMV serology: IgM absence. Elevated IgG: 369 u.
Virology: Diagnostic confirmation: positive PCR-CMV in saliva and urine [2].
Maternal CMV serology: IgM absence. Elevated IgG: 369 U.
Imaging
Cardiac ultrasound: absence of myocarditis.
Abdominal ultrasound: spleen present: 5 cm.
Thoracic scanner:
Bilateral bronchopneumopathy [3] (Figure 1).
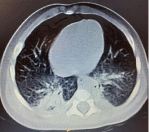
Figure 1: Chest scanner.
Immune assessment
Weight determination of immunoglobulins IgG, IgA, IgM, IgE. Assay of serum complement, CH50, C2, C3.Lymphocyte typing. Lack of immune deficiency.
Treatment
Nasal oxygen thérapy.
Oral Valganciclovir at a dosage of 16 mg/kg twice daily during 2 weeks [4].
In total
Clinical picture of pneumonia and monocnucleosis syndrome with thrombocytopenia prompted testing for CMV in saliva and urine. PCR + and favorable pulmonary and hematologic evolution under antiviral treatment lead to a diagnosis.
Discussion
Human cytomegalovirus is the most common congenital infection. About 1 to 3% of newborns become infected during pregnancy. Around 10 to 15% of the infants born after maternal seroconversion are symptomatic, and less than 3% after reactivation. The main reason for prenatal CMV transmission is primary infection of mothers during pregnancy rather than recurrent infection. Of even higher epidemiological relevance, however, is postnatal mother-tochild transmission, with breastfeeding being the main reason. In different studies, virolactia, or viral DNA in breast milk, was detected in 13 to 70% of lactating mothers. Using highly sensitive methods like PCR to screen for viral DNA in breast milk or cell-free milk whey, it has been demonstrated that 40% to 96% of seropositive mothers shed the virus via their breast milk. The reason for DNA and viruses in breast milk was the réactivation of CMV infections of the mothers during lactation.
To date the only confirmed sites of CMV latency in humans are undifferentiated progenitor cells of monocytes/granulocytes/ dendritic cells in the bone marrow.
Systemic inflammation and stress are the two known mechanisms triggering CMV reactivation from latency
The human breast harbors latently infected cells or latently infected cells are transported very efficiently to the lactating breast.
In the lactating breast, bioactive substances can be produced or accumulated which induce or support the replication of the virus in its target cells. Using in vitro transfection and infection systems, we have recently been able to show that cell-free milk whey is indeed capable of stimulating the gene expression of the CMV IE1/2 enhancer/promoter in monocytic cells (a possible target cell type for CMV in breastfeeding mothers. Breast). This promoter is responsible for the initiation of viral replication and, moreover, determines the efficiency of replication.
CMV was present in the respiratory tract of all infected infants [5].
In addition, milk whey enhanced replication of CMV in permissive human embryonic lung fibroblasts [6].
Apart from congenital CMV infection, CMV infection can be passed to newborn babies through breast milk. Reactivation in CMVpositive women is localized and limited in the breast. The acquisition of CMV via breast milk follows a trans-mucosal pathway.
Postnatal infection can be serious in premature babies because they lack the IgGs from their mothers, which are not transmitted until after 28 weeks of gestation. Postnatal infection through breast milk is often asymptomatic. However, it can occur noisily, especially in the presence of congenital immune deficiency. Pasteurization of breast milk is the most reliable method of eliminating CMV, but it changes the nutritional qualities of the milk. It is recommended in France for premature GA babies of less than 32 weeks. Freezing milk reduces the risk without eliminating it completely, but it does not change the qualities of the milk [7].
In term newborns it is transmitted to the oropharyngeal or nasopharyngeal level while in premature babies who are supplied with enteral nutrition CMV is transferred directly through the lining of the small intestine [8].
It is favored by stress, stimulation of interleukin II-6. Macrophage cells in breast milk are responsible for reactivating CMV. Transmission is possible from the first week of lactation and peaks between 4 and 8 weeks and would end at 3 months of lactation [9].
In this case report, reactivation could be favored by infection of the breast by Staphylococcus Aureus and by the two surgical interventions required as well as by the continuation of breastfeeding during the two months which separated the two surgical interventions inflammation, dysimmunity, phenomena favoring excretion CMV.
Conclusion
This case report shows that the morbidity of postnatal transmission of CMV in the term newborn is not always minimal. It should be taken in consideration that CMV becomes reactivated in all latently infected mothers during lactation and is shed within the breast milk.
References
- Nicoux M, Peterman L, Parodi M, Magny JM. Outcome and management of newborns with congenital cytomégalovirus infection. Arch Pediatr. 2020; 27: 160-165.
- Razonable RR, Inoue N, Pinninti SG, Boppana SB, Lazzarotto T, Gabrielli L, et al. Clinical diagnostic tests for human cytomegalovirus infections. J Infect Dis. 2020; 221.
- Pham A, El Mjati H, Nathan N, Kieffer F, Mitanchez D. Congénital cytomégalovirus infection manifesting as néonatal respiratory distress. Arch Pediat. 2017; 24: 872-876.
- Kadambari S, Whittaker E, Lyall H. Postnatally acquired cytomégalovirus infection in extremely prémature infants: how best to manage. Arch Dis Child Fetal and Neonatal Ed. 2020.
- Park HW, Cho MH, Bae SH, Lee R, Kim KS. Incidence of Postnatal CMV Infection among Breastfed Preterm Infants: a Systematic Review and Metaanalysis. J Korean Med Sci. Mars. 2021; 36: e84.
- Lazar K, Rabe T, Goelz R, Hamprecht K. Reactivation of human cytomegalovirus during lactation: impact of antibody kinetics and neutralization in blood and breast milk. Nutrients. 2020; 12: 338.
- Meier J, Lienicke U, Tschirch E, Krüger DH, Wauer RR, Prösch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005; 43: 1318-1324.
- Garofoli F, Civardi E, Zanette S, Angelini M, Perotti G, Zecca M, et al. Literature Review and an Italian Hospital Experience about Post-Natal CMV Infection Acquired by Breast-Feeding in Very Low and/or Extremely Low Birth Weight Infants. Nutrients. 2021; 13: 660.
- Bardanzellu F, Fanos V, Reali A. Human breast Milk acquired cytomégalovirus infection : certainties, doubts and perspectives. Curr Pediatr Rev. 2019; 15: 30-41.