
Research Article
J Bacteriol Mycol. 2021; 8(4): 1179.
Cryptococcus neoformans Virulence Attributes can be Modulated by Sound Stress
Kellysson GB Mendes1, Fabiana Brandao AS2*, Raul Alberto Laumann3 and Simoni Campos Dias1,4
1Catholic University of Brasilia, Proteomic and Biochemical Analysis Center, Brasilia-DF, Brazil
2University of Brasilia, Department of Pharmacy, Laboratory of Clinical Analysis, Darcy Ribeiro Campus, Asa Norte, Brasilia-DF, 70910-900, Brazil
3Embrapa Genetic Resources and Biotechnology, PqEBAv. W3 Norte (Final) S/N-Asa Norte, Brasilia-DF, 70770-90170770-917, Brazil
4University of Brasilia, Post-Graduation in Animal Biology, Darcy Ribeiro Campus, Asa Norte, Brasilia-DF, 70910-900, Brazil
*Corresponding author: Fabiana Brandao AS, University of Brasilia, Department of Pharmacy, Laboratory of Clinical Analysis, Darcy Ribeiro Campus, Asa Norte, Brasilia-DF, 70910-900, Brazil
Received: May 17, 2021; Accepted: July 01, 2021; Published: July 08, 2021
Abstract
Sound waves are a prime component making up the environment, and they are present in almost all niches on the planet. In times of increasing noise pollution, the effect of sound stress on humans, animals, and microorganisms is well known. However, the possibility of this kind of pressure in the environment, affecting pathogenic fungi, which live in the background as saprophytes, has not been explored. Fungi can develop attributes and become virulent due to adaptation to selective pressure or stress. In this context, our group has become interested in evaluating the impact of sound stress on the fungus Cryptococcus neoformans, a pathogen that has high phenotypic plasticity. C. neoformans strain H99 was chosen for all assays. The yeasts were cultivated at 30°C, exposed or not to the frequency of 8 kHz. We observed morphological changes in these cells, such as the expression of phenotype virulence attributes: capsule expansion and melanin production. We also analyzed the number of viable cells after exposure, and we observed the yeast’s susceptibility to antifungals. After the treatment with 8 kHz, the cells showed a significant increase in the capsule expansion, an acceleration of the melanin production, and a slight reduction in the number of viable cells. Finally, tests performed with the antifungals showed a decrease in inhibition halo on the plate test. Our results are innovative and suggest that stress caused by sound could incite increased virulence in this fungus.
Keywords: Cryptococcus neoformans, Sound frequency, Virulence Attributes, Stressor environment, Phenotypic Plasticity.
Introduction
Sound is mechanical energy that disperses in the form of waves, an intrinsic component of the environment [1,2]. From a human perspective, the sound frequencies can be divided into roughly three groups: infrasound (< 20 Hz), audible sound (20 to 20,000 Hz), and ultrasound > 20,000 Hz) [3].
Essentially, all life on the planet interacts with sound waves [4,5]. These interactions can be classified into two groups: 1- Interactions with sound self-produced by the organisms; these intentional interactions are usually involved in organism communication (5), and 2- Interactions that are non-intentional, when the organisms are exposed to environmental noise. The second group can have a major impact on living organisms, which vary according to the frequency range utilized and the organism that is exposed. It has been observed that plants exposed to the frequencies of 1 kHz significantly increased cell division and cell wall fluidity [6-8].In animals, the principal impact is induced by the sound in the audible sound frequencies, causing disorientation that disturbs the ability to communicate and hunt, and spatial orientation [9-12].
Hypotheses about the effect of global warming-related changes [13], radiation[14-16]and the use of pesticides [17] performing as selective pressure on pathogenic fungi in the environment have been raised. Besides, since the industrial revolution, the amount of noise emitted into the environment has significantly increased, considered today to be a problem only exceeded by pollution of air and water, especially in densely populated areas with intense industrial activity [12,18,19].
There is some evidence that sound, as environmental noise, could interfere in microorganism physiology,and this knowledge has been explored for decades in medicine [20-22]. Microbes growing exposed to different sound frequencies demonstrated significant changes such as increased cell permeability, change in cell surface charge, the release of nitric acid, hydrogen, peroxidase, and free radicals [22].
The effect of sound waves on the frequency of 8 kHz was investigated in the E. coliK-12 bacterial model. First, the researchers noticed an acceleration in RNA and protein synthesis, suggesting a periodic oscillation of the bacterial intracellular liquid induced by sound stress [3,23]. In a second experiment, it was shown that sound could induce mechanical stress, causing an influx of small molecules like H2O, Na+, K+, and Ca2+ [23]. A frequency-dependent fungicidal activity was observed in Aspergillus sp. [24-26].
Fungi are ubiquitous microorganisms in nature and have essential functions for maintaining life on Earth. However, some species are highly pathogenic, which is a result of a well-adapted selection of survival and infection in mammalian cells. Further, with the advent of immunosuppression, the number of fungal infections has increased significantly during the last few decades, reaching an alarming threshold of human mortality worldwide, affecting more than a billion people [27,28]. Questions are raised about what would happen in an environment that could promote selection for fungi strains that become increasingly virulent and resistant to the antifungals, and how this adaptation is also emerging at an exponential rate [29-31].
Some pathogenic fungi, such as Cryptococcus neoformans, present a saprophytic life cycle [32-34], and hypotheses discuss the ability of these pathogens to face environmental stresses, and how these events could modulate virulence factors [35-38]. These in turn could result in a more beneficial adaptation of these microorganisms when in the host’s infectious processes [38].
C. neoformans are encapsulated, polysaccharide-coated yeasts frequently found in the environment in association with decaying vegetation and are able to cause disease in humans [34,39,40]. This fungus can invade the Central Nervous System (CNS), causing fungal meningoencephalitis, which is the most common cause of meningitis in adults living with HIV in sub-Saharan Africa [41-43]. The global incidence of cryptococcal meningitis was recently estimated at approximately 220,000 per year [42]. The mortality for those receiving care was estimated at 60% in low-income countries [42,44]. People with compromised immune systems, especially those with AIDS and organ transplants are more susceptible to Cryptococcus infections [45-47]. In addition to its clinical importance, this fungal pathogen displays remarkable phenotypic plasticity in response to host and environment [36,48].
C. neoformans infects a wide range of organisms, from amoebas to insects (Lepidoptera) and plants such as Arabidopsis thaliana[49]. Hypotheses about the effect of global warming-related changes [13], radiation [14-16] and use of pesticides [17] could play a role as a form of selective pressure upon pathogenic fungi in the environment. In a broad view of the types of environmental stresses that would act as selective pressure, it is worth noting that since the industrial revolution, the amount of noise emitted into the environment has significantly increased. It is considered today as a problem on the scale of pollution of air and water, especially in densely populated areas with intense industrial activity [12,18,19].
In the face of this new concern about the role of sound/vibration in the environment, Biotremologyhas arisen as a new science. Biotremology is an emergent discipline that studies the production, transmission, reception, and biological effects of vibrations in a living organism [50,51]. The findings of this new science support studies in several domains, from animal communication to use in growth and pest control [52,53]. In this innovative vision, we investigate the effect of background noise as a source of substrate vibrations on the virulence attributes of the pathogen C. neoformans. The sound frequency of 2 and 8 kHz were evaluated on yeast growth, expansion of the polysaccharide capsule, melanin production, and susceptibility to fluconazole. Our data imply that sound can exert selective pressure on the environment, stimulating micro-organism virulence phenotypes.
Materials and Methods
Yeast strains and growth conditions
In our assays, we used the species Cryptococcus neoformansvargrubii, a well-established pathogenic strain, H99, a widely known virulent strain, with its entire genome sequenced [54]. Fungal strains were stored in 15% glycerol at -80°C until use. The cells were grown in YPD broth (yeast extract [2%], peptone [1%], dextrose [2%]) at 30°C and isolated in the log-phase of microbial growth for further testing.
Background Sound playback
A loudspeaker (of low-frequency response, 8O impedance, membrane diameter 10 cm, Radioshack, Taiwan)was usedfor the emission of the sound waves. Over the speaker was placed a 15 cm acrylic plate, on which the Petri dishes with fungal strains were arranged, produced as described in the previous section, were placed. With this setup, the sound was transmitted as a substrate vibration of the acrylic plate to the Petri dishes.
For the experiments, the complete setup was introduced in a cultivation oven that maintained the temperature at 30°C and alsoacted as an isolation sound chamber.
Two simulation programs were built, using the function synthesis of the software Sound Forge 6.0 software (Sonic Foundry Inc., Madison, WI, U.S.A.). The programs consisted of digital pure tone continuous sequences of 2 or 8 kHz frequencies built in monophonic mode at 24-bit, 96-kHz, 80-dB signal-to-noise ratio. The stimulation program was played back without interruptions (looping reproduction mode of the Sound Forge software) during all duration periods of the experiments, using a computer connected to a sound card (UA-25EX, Edirol-Roland 24bits-96kHz; RolandCorp., Japan) which the loudspeaker was plugged into.
The measure of air sound intensity was assessed by a digital decibelimeter (minipa Model MSL-1355b, SPS, Brazil) connected to a computer and placed in the middle of the cultivation oven. Vibrations transmitted to the acrylic plate were recorded by a laser vibrometer (PDV-100, Polytec, Waldbronn, Germany). The beam of the vibrometer was directed perpendicularly to different points of the acrylic plate and the equipment was placed at a distance of ~30 cm from the vibration surface. To get a better reflection, a small piece of reflective tape was glued on the recording points. Registered signals were amplified and digitized (monophonic mode, 24-bit, 96-kHz, 100-dB signal-to-noise ratio),via the audio capture sound card described above, and computer-stored using Cool Edit Pro 2.0 software (Adobe Systems Inc., San Jose, CA, U.S.A.).
Airborne sound intensity inside the cultivation oven was 57.6 dB. The air pressure generated by the loudspeaker membrane was transmitted efficiently to the acrylic plate and to the Petri dish, Vibration generated by the air pressure varied between -1 and -21 dB, in relation to the intensity measured in the loudspeaker membrane (-50 dB) (Supplementary Figure 1).
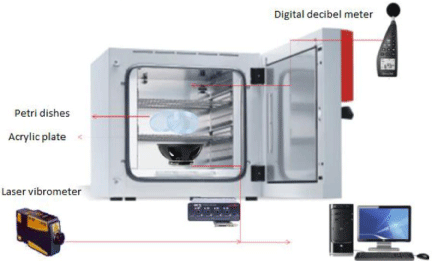
Figure 1: Schematic sound wave load apparatus. Sound wave apparatus adapted to an oven to produce an environmental sound stress.
Melanin production
Melanin production was assessed by inoculation of the cells in a chemically defined minimal medium (15 mM dextrose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine and 3 μM thiamine, supplemented with 1 mM L-DOPA (Sigma-Aldrich), Agar [1,5 %], pH 5.5) and incubated at 30°C. The daily evaluation was qualitative, classified only by the presence or absence of pigmentation of the colonies [36,55].
Capsule induction
To induce the capsule formation, yeast cells grown in YPD were washed 3 times with 1X Phosphate Saline Buffer Sterile (PBS) and the density was adjusted to 108 cells/mL. A 10μL-aliquot of yeast suspension was plated onto the five points of the Petri dishes containing a chemically defined minimal medium (15 mM dextrose, 10 mM MgSO4, 29.3 mM KH2PO4, 13 mM glycine and 3 mM thiamine, Agar [1.5%], pH5.5). After this, the plates were incubatedat 30°C, with no shaking. The control group was incubated without any frequency, and the test group was incubated under the 8 kHz frequency. Each day, colonies were scraped peripherally, and this scraping was transferred to a slide and stained for visualization and measurement of the capsule expansion.
India Ink staining and light microscopy analyses
For the capsule size measurement, 10 μL of induced yeast cells were mixed with an India Ink drop (Becton Dickinson, NJ) and observed under the light microscope (Axiovert 100) at a magnification of 35 x. At least 4 different fields were randomly chosen and photographed. To calculate the capsule size, the whole-cell diameter (yeast cell + capsule) and the cell body diameter (limited by the cell wall) were measured by the Axion VS 40 x 64 V 4.8.3.0 software (Carl Zeiss MicroImaging). Capsule thickness was defined as the difference between the whole cell diameter and the cell body diameter. For each observation, at least 50 cells were measured. The assays were performed in duplicate.
Cell viability assay
This was performed by counting Colony-forming Units (CFU) in order to evaluate the number of viable cells after the four days of 8 kHz treatment. After four days, the whole colony was removed from the plate and placed in 20 mL of sterile saline (0.9%). Next, the cells were homogenized and a dilution of 1:104 made. With the aid of a sterile Drigalski handle, 100 μL of cell suspension was scattered on the YPD plate. The plates were incubated in the oven for 48 h, at 30°C ± 2, and at the end of this period, a cell number was counted. Previous studies testing sound frequency effects on Aspergillus sp. and E. coli K-12 demonstrated that frequencies lower than 5 kHz (Aspergillus) and 8 kHz (E. coli) could safely be appliedto microorganisms without leading to cell death. (20,21) From this observation, we decided to test the frequency of 2 kHz and 8 kHz in C. neoformans.
Antifungal susceptibility
Two antifungals, Fluconazole and Amphotericin B, were used in this assay, both of which are clinical antifungal treatments for cryptococcosis[56,57]. We applied the disk diffusion technique on Sabouraud agar, with a disk positioned in the center of the Petri dish and containing 20 μL of fluconazole prepared with 2mg/mL, or the antifungal disk immersed in Amphotericin B (250 μg/mL). The yeast cell density spread on the plate was set at between 0.5 and 1.0 on the McFarland scale.
Statistical analysis
Data are expressed as means of at least triplicate samples. Statistical analysis was performed by using GraphPad Prism version 7.0 for Mac (GraphPad Software). All tests were conducted at a significance level of p<0.05. Normality assumptions were verified applying the Shapiro-Wilktest. The Student test was applied when two groups were compared, the control wild-type versus test groups. The 95% confidence interval was determined for all the experiments.
Results
The effects of sound-wave stimulation on virulenceassociated phenotypes
The treatment performed with the frequency of 8 kHz demonstrated an acceleration in melanization within the first 24 hours of growth, comparing the control group and the test group, given the darkening of the test colonies (Figure 2). The darkening of the fungal spots is directly related to the melanin accumulation produced by fungal cells. This acceleration of the darkening was more visible on the first two days of growth; after that, the colonies reach the plateau of melanization, and it became impossible to notice any further darkening between the groups (Figure 2).
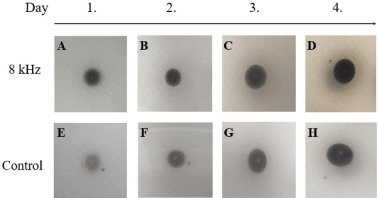
Figure 2: C. neoformans melanization is accelerated by 8 kHz frequency. C. neoformans strain H99 cells were grown overnight and washed 3 times, and 106
yeast were spotted on a solid minimal medium supplemented with L-DOPA. The colonies were followed visually for melanin production for 4 days. We observed that
in cultures treated with 8 kHz frequency, the acceleration of the darkening was remarkable from the first 24 hours (A) and lasted until the plateau at day 4 (B-D).
The control in MM started to show an evident pigment from day 2 (F-H).
The 2 kHz frequency displayed inverse results, with the test group demonstrating a slower melanization than that presented by the control group, with the darkening of the test group colonies noticeable only on the third day (Supplementary Figure 2). Importantly, even after four days of growth under 2 kHz, the darkening of the test cells was reduced when compared to the control cells.
The effect of sound frequency on the capsule size
Since only the 8 kHz was able to affect melanin production, we decided to investigate this frequency using other virulence phenotypes. The capsule is frequently mentioned as the main C. neoformans virulence factor [48,58-61], and in situations of cellular stress, the fungus expands its capsule [62,63].
Accordingly, we decided to investigate if the 8 kHz sound frequency could cause stress that would lead to the capsule expansion. The average capsule size was measured daily throughout the growth period, with the recovered values being plotted in graphs presented in Figure 3. It was observed that the group submitted to sound treatment showed a larger average size of both cell body and capsule (C+C 8 kHz) compared to the control group (C+C control) (Figure 3A). When we analyzed only the cell body, we observed an increasein the size of the test group (Cell 8 kHz), measuring approximately 10 to 15 micrometers compared to control (Figure 3B). Analyzing only the capsule ((C+C) - (Cell), we also observed a significant increase in the capsule expansion, with relatively higher valuesin the group exposed to 8 kHz frequency (Figure 3C).
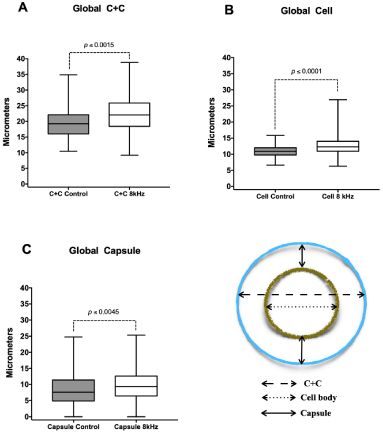
Figure 3: Analysis of the effect of sound frequency on C. neoformans capsule expansion. Capsule sizes, bodies, and total cell diameters of C. neoformans
H99 cells were determined by light microscopy under India ink staining. Daily yeast samples were taken from colonies grown on the test and control plates. The
global averages of daily analysis are represented in the graphs. C + C (Cell + Capsule), Cell (only cell body, without capsule), capsule (only Capsule, without cell
body). The boxes represent 75 percent of the data distribution; the horizontal lines represent the means. The bars indicate the maximum and minimum values.
Statistical tests: t student.
Cell viability and 8 kHz interaction
Due to the observations of the sound effect on theC. neoformans virulence factors, we decided to investigate whether the 8 kHz frequency would interfere with cell viability. The CFU test was carried out at the end of the tests, after four days of exposure to sound frequency. The entire colonies were removed from the plate, and the cell density was adjusted with the aid of the Neubauer chamber.
CFU counts (Figure 4) demonstrated that the 8 kHz frequency did not affect the yeast cell’s viability.
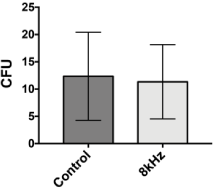
Figure 4: Analysis of cell viability after sound treatment. The CFU
analyses were performed in triplicate using the colonies grown on the test and
control plates. Colonies were removed with the aid of a sterile spatula and
homogenized in 20 mL of sterile saline. Then 50 μL of the suspension was
spread on a Petri dish with Sabouraud Agar medium and incubated at 30°C
for 48 h, for later counting of colony-forming units. Bars representa mean
values and lines SE. Statistical test: t student.
Antifungal susceptibility
Due to the effects caused by the frequency of 8 kHz over the melanin and capsule phenotypes, which are essential for the establishment of the disease in humans, we decided to investigate whether this sound stress could also interfere with resistance to clinical antifungals. Using the disk-plate diffusion technique, we evaluated the effect of the 8 kHz frequency on C. neoformans when subjected to the membrane-targeting antifungals Fluconazole and Amphotericin B.
Fluconazole (FLU) is an antifungal that interferes with the ergosterol biosynthetic pathway by inhibiting Erg11, an important component of the ergosterol biosynthesis pathway [64]. In our results, we observed a reduction in the size of the Flu inhibition halo, when yeasts were exposed to 8 Khz frequencies compared to control without exposure (Figure 5A and 5B).
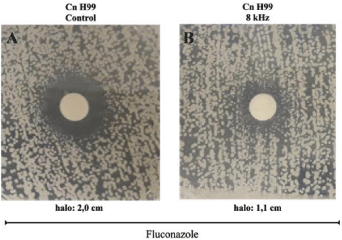
Figure 5: Membrane-targeting antifungal is less active at 8 kHz sound effects. Assessing MICs and the zones of inhibition of membrane-targeting Fluconazole
cells grown on YPD agar. Disks containing 10 μg of Fluconazole were placed over the top of the agar. Cells were incubated for 48 h at 37°C. The 8 kHz frequency
reduced the halo of yeast inhibition by 0.9 cm (B-1.1 cm halo) compared to control (A -2.0 cm halo).
Amphotericin B (AMB) is a polyene antifungal that influences the breakdown of membranes differently from FLU. AMB removes ergosterol from fungal membranes [65]. Our data showed no difference between the AMB halo inhibition of the control and the test (Supplementary Figure 3).
Discussion
8 kHz sound frequency effect on C. neoformans melanization
Melanin is synthesized by the fungus in order to assist the microorganism in its survival mechanisms, conferring some resistance to environmental conditions such as pH [66,67], radiation [14,15], high temperatures [68], oxidative stress [61], and other stressors present in the environment in which the fungus is growing [69,70].
The ability to produce melanin is fundamental to the virulence of C. neoformans, whereas non-melanin-producing mutant strains significantly lose their virulence[61,66,71]. Given that melanin production is a protective factor against environmental stresses, we hypothesized that if the sound caused stress on the fungus, melanin production could be affected. Interestingly, our data reveal that the 2 kHz frequency transmitted to the fungal colony as a vibration subtly delayed melanin production, followed by colony darkening. On the other hand, the 8 kHz frequency induced an acceleration in melanin production, and the test group became darker faster than the control group.
The sound frequencies generated by the frequency of 8 kHz upon reaching the cell most likely resulted in the excitement of molecules intracellularly, causing a response that accelerated melanin production. It is reasonable to consider that it may have been an increase in the expression of genes responsible for melanin synthesis, since a similar effect was observed in the bacteria E. coli K-12 [23], and in plants [8,72]. In E. coli K-12 exposed to 8 kHz, an increase was observedin the intracellular protein concentration and the synthesis of RNA, in the early treatment stages, which was in favor of cell division [23].
Cell population density is a crucial point for melanin production [73]. Hence, as an increase in cell division of E. coli treated with 8 kHz was observed, we decided to appraise whether the effect on the acceleration of melanin production was due to an increase in population density, due to a gain in cell division. As both groups were inoculated with the same fungal density (108 yeast/mL), it is possible to discard the possibility that the control group produces melanin at a slower rate due to decreased fungal density. Our data on Cell- Forming Colony Units (CFU) demonstrated that the population in the spots of the test group showed a slight reduction, although it was not statistically significant. These data suggest that there was no increase in population density in the group treated with 8 kHz compared to the control group. Therefore, the acceleration effect on melanin production is related to the stress caused by the sound wave on the expression of this virulence phenotype.
Curiously, the signals of cellular stress observed at 8 kHz treatment did not reproduce in yeasts treated with lower frequencies, such as 2 kHz. This corroborates previous studies that have indicated that the interactions between the sound frequencies and organisms vary according to the applied frequency, with some studies indicating that they explore the higher frequency as a prominent potential stressor [23].
C. neoformans cell body size and capsule expansion are affected by sound waves
Unicellular organisms can expand their body cell size as an adaptive response to unfavorable environmental conditions [74]. Gu et al. [23] observed that the sound intensity level of 100 dB increased E. coli K-12 length bymore than 27.26% [23]. Cell body size is an important adaptive feature with a direct influence on all physiological cell mechanisms, such as RNA and protein synthesis [23]In our analysis,C. neoformans body cell size was most affected by the sound waves, becoming larger than the control group cell bodies. In cell surface phenotypes, Pelling et al. [75] observed that theSaccharomyces cerevisiae cell wall exhibited periodic nanoscale motions in an acoustically insulated environment [75]. Here, we detected a significant increase in capsule expansion in the test group (8 kHz). The capsule, for several authors, is the most relevant C. neoformans virulence factor [48,58,61,76]. It gives the fungus resistance against environmental stressors such as dehydration [77-79] and free radicals [80], and despite this important role in Cryptococcus virulence [60], the capsule is not essential for fungal growth [76]. Nor is melanin, since mutant strains that do not produce these phenotypes can replicate normally,although they become less virulent [36,48,81,82].
Together, these observations corroborate the hypothesis that sound waves can stressC. neoformans yeast cells in the environment, since these phenotypes are cells’ responses to adaptive factors. These results reinforce the hypothesis that noise stress can also play an important role in triggering or even “training” the virulence factors.
8 kHz does not affect cell viability
The number of viable cells was slightly reduced after treatment, although without statistical significance. However, a reduction in the number of viable cells corroborates previous results and the indication that the sound frequency acts as a stressor component, and these results are compatible with those obtained by Karippen et al., with Aspergillus, indicating that the higher the frequency applied the higher its fungicidal potential [24]. This result suggests that the effects observed on the virulence phenotypes are caused by the sound wave, but without affecting the yeasts’ viability.
Increasing resistance to fluconazole
Antifungal resistance is becoming a significant concern for patients at high risk from invasive mycoses. Treatment options for invasive fungal infections are limited [31].Cryptococcosisis treated with amphotericin B, combined with fluconazole and/or 5-flucytosine [83]. As previously mentioned in this work, in recent years it has been proposed that environmental pressures affect the virulence of Cryptococcus spp., as well as their susceptibility to clinical drugs [84,85].
Previous data have already shown that sound affects the membrane fluidity and that it increased under sound stimulation of some strength and frequency [86-88]. Interestingly, in our analyses testing antifungals that affect the plasma membrane, by different routes, we observed that 8 kHz sound frequency was capable of a slight increase in the C. neoformans fluconazole resistance, but not in Amphotericin B. It is very likely that the vibration caused by the cell interaction with the sound causes intercommunication among molecules inside the yeast cell. Although more examinations are required to test this hypothesis, it seems reasonable to consider it at this point, since the results of sound interaction demonstrated this effect in E. coli[24]. Furthermore, no effect was observed for Amphotericin B, which presents a mechanism related to the breakdown of membranes, more outside, while fluconazole acts in biosynthesis, which is inside.
Nevertheless, due to the increased expression of the virulence phenotypes, such as melanin and capsule, we were expecting to notice no sound effect upon antifungals’ interaction. We believe that the stress factor caused by sound waves only acts to boost the resistance already present in the strains, and does not confer a new resistance.
In summary, during the experiments, the presence of a possible interaction between the sound waves and C. neoformans virulence factors was noted (Figure 6). Our data are unprecedented regarding sound effects on virulence and adaptation of the opportunistic pathogen C. neoformans, a pathogen leading to the highest number of cases of morbidity/mortality in HIV patients. We be certain of that this work has pointed to a new landscape for sound frequencies as an intrinsic component in the environment, playing a role in microorganism adaptation and virulence process. This may contribute to extensive understanding of biological processes and possibilities for biomedical approaches in the future.
Figure 6: Model for Sound stress on the main C. neoformans virulence phenotypes. Cells sense environmental stress, such as 8 kHz sound waves, and a
signal is transmitted into the cell. The signal results in an adaptive response that requires regulation of the virulence phenotypes (acceleration in melanin production,
increased cell body and capsule, and increased resistance to Fluconazole) as demonstrated in the scheme.
Conclusion
Sound can have multifaceted effects on microorganisms, and there may be frequencies that slow or accelerate microbial growth. Our data suggest that noise could act as a stressor on C. neoformans and may result in a gain in virulence expression. The collective forces of acquired virulence factors enabled this fungus to bypass and elude the mammalian immune response during infection, leading to the latency and persistence of cryptococcal infection.
After these data, our group is now interested in further investigating these effects, since specific sound frequencies that stimulate cell growth can assist in the efficiency of diagnosis using the cultivation of fastidious microorganisms. On the other hand, inhibiting growth or causing cell damage can lead to ways to prevent microorganisms from forming biofilms on biotic and abiotic surfaces.
References
- Alías F, Alsina-Pagès RM. Review of Wireless Acoustic Sensor Networks for Environmental Noise Monitoring in Smart Cities. Journal of Sensors. 2019.
- Danet AF, Cheregi MC, Badea M. Environmental Pollution Monitoring: Laboratory Guide. Pro Act Birotic. 2005.
- Shaobin G, Wu Y, Li K, Li S, Ma S, Wang Q, et al. A pilot study of the effect of audible sound on the growth of Escherichia coli. Colloids Surf. B Biointerfaces. 2010a; 78: 367-371.
- Michelsen A. Hearing and Sound Communication in Small Animals: Evolutionary Adaptations to the Laws of Physics, in: Webster, D.B., Popper, A.N., Fay, R.R. (Eds.), The Evolutionary Biology of Hearing. Springer New York, New York, NY. 1992; 61-77.
- Myrberg AA. Sound Communication and Interception in Fishes, in: Hearing and Sound Communication in Fishes. Springer New York. 1981; 395-426.
- Choi B, Ghosh R, Gururani MA, Shanmugam G, Jeon J, Kim J, et al. Positive regulatory role of sound vibration treatment in Arabidopsis thaliana against Botrytis cinerea infection. Sci Rep. 2017; 7: 2527.
- Fernandez-Jaramillo AA, Duarte-Galvan C, Garcia-Mier L, Jimenez- Garcia SN, Contreras-Medina LM. Effects of acoustic waves on plants: An agricultural, ecological, molecular and biochemical perspective. Sci Hortic. 2018; 235: 340-348.
- Hassanien RHE, Hou T, Li Y, Li B. Advances in effects of sound waves on plants. J. Integr. Agric. 2014; 13: 335-348.
- Bowles A. Schulte-Fortkamp B. Noise as an Indicator of Quality of Life: Advances in Measurement of Noise and Noise Effects on Humans and Animals in the Environment. Acoustics Today. 2008.
- Brown AL. Measuring the effect of aircraft noise on sea birds. Environ Int. 1990; 16: 587-592.
- Elemans CPH, Rasmussen JH, Herbst CT, Düring DN, Zollinge, SA, Brumm H, et al. Universal mechanisms of sound production and control in birds and mammals. Nat Commun. 2015; 6: 8978.
- Jhanwar D. Noise Pollution: A Review. Journal of Environment Pollution and Human Health. 2016; 4: 72-77.
- Casadevall A, Kontoyiannis DP, Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. MBio. 2019b; 10.
- Casadeval A, Cordero RJB, Bryan R, Nosanchuk J, Dadachova E. Melanin, Radiation, and Energy Transduction in Fungi. The Fungal Kingdom. 2017; 509-514.
- Dadachova E, Casadevall A. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008; 11: 525-531.
- Tugay T, Zhdanova NN, Zheltonozhsky V, Sadovnikov L, Dighton J. The influence of ionizing radiation on spore germination and emergent hyphal growth response reactions of microfungi. Mycologia. 2006; 98: 521-527.
- Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, et al. Genomic Context of Azole Resistance Mutations in Aspergillus fumigatus Determined Using Whole-Genome Sequencing. MBio. 2015; 6: e00536.
- Brown AL, Lex Brown A. Effects of Road Traffic Noise on Health: From Burden of Disease to Effectiveness of Interventions. Procedia Environmental Sciences. 2015.
- Brown A, van Kamp I. WHO Environmental Noise Guidelines for the European Region: A Systematic Review of Transport Noise Interventions and Their Impacts on Health. International Journal of Environmental Research and Public Health. 2017.
- Goncarovs KO, Miskovic Feutz M, Perez-Moreno C, Couetil LL. Efficacy and safety of sound wave treatment of recurrent airway obstruction in horses. J Vet Intern Med. 2010; 24: 1503-1508.
- Levy Y, Agnon Y, Azhari H. Measurement of speed of sound dispersion in soft tissues using a double frequency continuous wave method. Ultrasound in Medicine & Biology. 2006.
- Rokhina EV, Lens P, Virkutyte J. Low-frequency ultrasound in biotechnology: state of the art. Trends Biotechnol. 2009; 27: 298-306.
- Gu S, Zhang Y, Wu Y. Effects of sound exposure on the growth and intracellular macromolecular synthesis of E. coli k-12. PeerJ. 2016; 4: e1920.
- Karippen PM, Dayou J, Phin CK. Experimental investigation on the effects of audible sound to the growth of Aspergillus Spp. Modern Applied Science. 2009; 3: 137-141.
- Mantas P, Pagan R, Raso J. Predicting Lethal Effect of Ultrasonic Waves Under Pressure Treatments on Listeria monocytogenes ATCC 15313 by Power Measurements. J Food Sci. 2000; 65: 663-667.
- Shaobin G, Wu Y, Li K, Li S, Ma S, Wang Q, et al. A pilot study of the effect of audible sound on the growth of Escherichia coli. Colloids Surf. B Biointerfaces. 2010b; 78: 367-371.
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden Killers: Human Fungal Infections. Science Translational Medicine. 2012.
- Tudela JLR, Denning DW. Recovery from serious fungal infections should be realisable for everyone. Lancet Infect. Dis. 2017; 17: 1111-1113.
- Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017; 17: e383-e392.
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019; 25: 792-798.
- Revie NM, Iyer KR, Robbins N. Cowen LE. Antifungal drug resistance: evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018; 45: 70-76.
- Chowdhary A, Rhandhawa HS, Prakash A, Meis JF. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: an update. Crit. Rev. Microbiol. 2012; 38: 1-16.
- Harrison TS. Cryptococcus neoformans and cryptococcosis. J Infect. 2000a; 41: 12-17.
- Springer DJ, Billmyre RB, Filler EE, Voelz K, Pursall R, Mieczkowski PA,et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: identification of the local environmental source as arboreal. PLoS Pathog. 2014; 10: e1004285.
- et al. A hidden battle in the dirt: Soil amoebae interactions with Paracoccidioides spp. PLoS Negl. Trop. Dis. 2019; 13: e0007742.
- Brandão F, Esher SK, Ost KS, Pianalto K, Nichols CB, Fernandes L, et al. HDAC genes play distinct and redundant roles in Cryptococcus neoformans virulence. Sci Rep. 2018; 8: 5209.
- Casadevall A, Steenbergen JN, Nosanchuk JD. “Ready made” virulence and “dual use” virulence factors in pathogenic environmental fungi-the Cryptococcus neoformans paradigm. Current Opinion in Microbiology. 2013.
- Elías-Villalobos A, Fernández-Álvarez A, Moreno-Sánchez I, Helmlinger D, Ibeas JI. The Hos2 Histone Deacetylase Controls Ustilago maydis Virulence through Direct Regulation of Mating-Type Genes. 2015.
- Harrison TS. Cryptococcus neoformans and Cryptococcosis. Journal of Infection. 2000b.
- Litvintseva AP, Carbone I, Rossouw J, Thakur R, Govender NP, Mitchell TG. Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS One. 2011; 6: e19688.
- Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010; 10: 67.
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. The Lancet Infectious Diseases. 2017a.
- Siddiqi OK, Ghebremichael M, Dang X, Atadzhanov M, Kaonga P, Khoury MN, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis. 2014; 58: 1771- 1777.
- Mourad A, Perfect JR. Present and Future Therapy of Cryptococcus Infections. J Fungi (Basel). 2018; 4.
- Baddley JW, Schain DC, Gupte AA, Lodhi SA, Kayler LK, Frade JP, et al. Transmission of Cryptococcus neoformans by Organ Transplantation. Clin Infect Dis. 2011; 52: e94-e98.
- Marinelli T, Anagnostou N, Daniel S, Wigg AJ, Teh J. Very early-onset of Cryptococcus neoformans disease following liver transplantation: Report of two cases and a review of the literature. Transpl Infect Dis. 2020; 22: e13227.
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. The Lancet Infectious Diseases. , 2017b.
- O’Meara TR, Alspaugh JA. The Cryptococcus neoformans Capsule: a Sword and a Shield. Clinical Microbiology Reviews. 2012.
- Zaragoza O. Basic principles of the virulence of Cryptococcus. Virulence. 2019; 10: 490-501.
- Hill PSM, Lakes-Harlan R, Mazzoni V, Narins PM, Virant-Doberlet M, Wessel A. Biotremology: Studying Vibrational Behavior. Springer International Publishing. 2019.
- Hill PSM, Wessel A. Biotremology. Curr Biol. 2016; 26: R187-R191.
- Laumann RA, Bottura DH. Use of vibratory signals for stink bug monitoring and control. Stinkbugs: Biorational Control Based on Communication Processes. 2017.
- Polajnar J, Eriksson A, Lucchi A, Anfora G, Virant-Doberlet M, Mazzoni V. Manipulating behaviour with substrate-borne vibrations--potential for insect pest control. Pest Manag. Sci. 2015; 71: 15-23.
- Janbon G, Ormerod KL, Paulet D, Byrnes EJ, Yadav V, Chatterjee G, et al. Analysis of the Genome and Transcriptome of Cryptococcus neoformans var. grubii Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation. PLoS Genet. 2014; 10.
- Brandão FAS, Derengowski LS, Albuquerque P, Nicola AM, Silva-Pereira I, Poças-Fonseca MJ. Histone deacetylases inhibitors effects on Cryptococcus neoformans major virulence phenotypes. Virulence. 2015; 6: 1-13.
- Perfect JR, Bicanic T. Cryptococcosis diagnosis and treatment: What do we know now. Fungal Genet Biol. 2015; 78: 49-54.
- Pianalto KM, Alspaugh JA. New Horizons in Antifungal Therapy. J Fungi (Basel). 2016; 2.
- Casadevall A, Coelho C, Cordero RJB, Dragotakes Q, Jung E, Vij R. The capsule of Cryptococcus neoformans. Virulence. 2019a.
- Cordero RJB, Pontes B, Guimarães AJ, Martinez LR, Rivera J, Fries BC. Chronological aging is associated with biophysical and chemical changes in the capsule of Cryptococcus neoformans. Infect. Immun. 2011; 79: 4990- 5000.
- García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One. 2011; 6.
- Heitman, Kozel, Kwon-Chung, Perfect, Casadevall (Eds.). Melanin: Structure, Function, and Biosynthesis in Cryptococcus, in: Cryptococcus. American Society of Microbiology. 2011; 55-66.
- Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog. 2011; 7.
- Jacobson ES, Tingler MJ, Quynn PL. Effect of Hypertonic Solutes Upon the Polysaccharide Capsule in Cryptococcus neoformans. Mycoses. 2009.
- Brown HE, Telzrow CL, Saelens JW, Fernandes L, Alspaugh JA. Sterol- Response Pathways Mediate Alkaline Survival in Diverse Fungi. MBio. 2020; 11.
- Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci USA. 2012; 109: 2234-2239.
- Jacobson ES, Tinnell SB. Antioxidant function of fungal melanin. J. Bacteriol. 1993; 175: 7102-7104.
- Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003; 38: 143-158.
- de Gontijo FA, Pascon RC, Fernandes L, Machado J, Alspaugh JA, Vallim MA. The role of the de novo pyrimidine biosynthetic pathway in Cryptococcus neoformans high temperature growth and virulence. Fungal Genet Biol. 2014; 70: 12-23.
- Cordero RJ, Casadevall A. Functions of fungal melanin beyond virulence. Fungal Biol Rev. 2017; 31: 99-112.
- Silva MB, Thomaz L, Marques AF, Svidzinski AE, Nosanchuk JD, Casadevall A, et al. Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Mem. Inst. Oswaldo Cruz. 2009; 104: 644- 648.
- Kwon-Chung KJ, Polacheck I, Popkin TJ. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982; 150: 1414-1421.
- Bochu W, Jiping S, Biao L, Jie L, Chuanren D. Soundwave stimulation triggers the content change of the endogenous hormone of the Chrysanthemum mature callus. Colloids Surf. B Biointerfaces. 2004; 37: 107-112.
- Albuquerque P, Nicola AM, Nieves E, Paes HC, Williamson PR, Silva-Pereira I, et al. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. MBio. 2013; 5: e00986.
- Turner JJ, Ewald JC, Skotheim JM. Cell size control in yeast. Curr Biol. 2012; 22: R350-R359.
- Pelling, A.E., Sehati, S., Gralla, E.B., Valentine, J.S., Gimzewski, J.K., 2004. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science. 2004; 305: 1147-1150.
- Cordero RJB, Bergman A, Casadevall A. Temporal behavior of capsule enlargement by Cryptococcus neoformans. Eukaryot. Cell. 2013; 12: 1383- 1388.
- García-Rodas R, Cordero RJB, Trevijano-Contador N, Janbon G, Moyrand F, Casadevall A. Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. MBio. 2014; 5: e00945.
- McFadden DC, De Jesus M, Casadevall A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem. 2006; 281: 1868-1875.
- Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009; 68: 133-216.
- Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodríguez-Tudela JL, et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008; 10: 2043-2057.
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994; 14: 4912-4919.
- Trevijano-Contador N, Rossi SA, Alves E, Landín-Ferreiroa S, Zaragoza O. Capsule Enlargement in Cryptococcus neoformans Is Dependent on Mitochondrial Activity. Front Microbiol. 2017; 8: 1423.
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010; 50: 291-322.
- Bastos RW, Carneiro HCS, Oliveira LVN, Rocha KM, Freitas GJC, Costa MC, et al. Environmental Triazole Induces Cross-Resistance to Clinical Drugs and Affects Morphophysiology and Virulence of Cryptococcus gattii and C. neoformans. Antimicrob. Agents Chemother. 2018; 62.
- Del Poeta M, Casadevall A. Ten challenges on Cryptococcus and cryptococcosis. Mycopathologia. 2012; 173: 303-310.
- Hu-Cheng Z, Bo-Chu W, Shao-Xi CAI, Bao-Shu XI. Effect of sound stimulation on the lipid physical states and metabolism of plasma membrane from chrysanthemum callus. J Integr Plant Biol. 2002; 44: 799-803.
- Zhao HC, Wang BC, Liu BA, Cai SX, Xi BS. The effects of sound stimulation on the permeability of K channel of Chrysanthemum Callus plasma. Colloids and Surfaces B: Biointerfaces. 2002.
- Levine ND. World Health Organization. 1979.Receptivity to Malaria and Other Parasitic Diseases of A Who Working Group.Who Regional Office For Europe, Copenhagen, Denmark. 1980.