
Research Article
J Bacteriol Mycol. 2021; 8(5): 1182.
Candida albicans Secreted Aspartic Protease 7 is Essential for Damage of Human Oral Epithelial Cells
Yang HP1,2, Tsang PCS3, Pow EHN4, Lam OLT5 and Tsang PWK6,7*
1The Affiliated Stomatology Hospital, Zhejiang University School of Medicine, China
2Key Laboratory of Oral Biomedical Research of Zhejiang Province, Zhejiang University School of Medicine, China
3Department of Operative Dentistry, Faculty of Dentistry, The University of Hong Kong, Hong Kong, China
4Department of Prosthodontics, Faculty of Dentistry, The University of Hong Kong, Hong Kong, China
5George and Fay Yee Centre for Healthcare Innovation, University of Manitoba, Canada
6School of General Education and Languages, Technological and Higher Education Institute of Hong Kong, Hong Kong, China
7Centre for Interdisciplinary Research on Food Byproducts Utilization, Technological and Higher Education Institute of Hong Kong, Hong Kong, China
*Corresponding author: Paul Wai Kei Tsang, Technological and Higher Education Institute of Hong Kong, Hong Kong, China
Received: June 02, 2021; Accepted: July 19, 2021; Published: July 26, 2021
Abstract
Aims: Candida albicans is an important human fungal pathogen in clinical settings. It possesses a wide spectrum of virulence traits, including but not limited to the production of Secreted Aspartic Proteases (SAPs), to invade host cells under predisposing conditions. The aims of the present study were to investigate the functional role of C. albicans SAP7 in invasion ability.
Methods: The present study was carried out to construct C. albicans sap7Δ/Δ mutant strain using a PCR-based gene disruption method. The behaviors of this SAP7 knockout strain was evaluated and compared with the wild type and SAP7 complemented strains between human oral epithelial cells with respect to endocytosis, invasion, and tissue damage.
Results: Compared with the wild type C. albicans strain, a 52% reduction in the endocytosis of the sap7Δ/Δ mutant strain by oral epithelial cells was observed, as well as a 25% attenuation of internalization, and a 27% reduction of tissue damage (P<0.05).
Conclusion: Our data clearly demonstrates that C. albicans SAP7 contributes to tissue invasion into human oral epithelial cells which warrant further investigations as potential targets for antifungal interventions.
Keywords: Candida albicans; Secreted aspartic protease; Oral epithelial cells; Endocytosis; Tissue damage
Introduction
Candida species are the major cause of opportunistic fungal infections in humans. They exist as harmless commensal in immunocompetent individuals and inhabit in human oral cavity, gastrointestinal and urogenital tracts [1]. However, under predisposing conditions, when normal microbiota is disturbed or immune system is compromised, Candida fungi transform into lethal microbes and cause superficial infections or deep-seated, systemic candidiasis [2,3]. In fact, candidiasis ranks fourth among the leading types of nosocomial infections in clinical sectors with high mortality and morbidity rates ranging from 40-60% in spite of careful management of antifungal interventions [4].
It has been considered epithelial tissues are the first line of defense of humans against pathogenic invasion and manifestations. In Candida fungi, two distinguished but complementary pathways of invasion to human oral epithelial cells exist: induced endocytosis and active penetration. The induced endocytosis of C. albicans is triggered by hyphae that the host cells produce pseudopods and engulf the microorganisms [5,6]. Two Candida invasins have been found: Agglutinin-like sequence 3 (Als3) and heat shock protein 1 (Ssa1) found on the surface of hyphae play a key role in induced endocytosis via binding to specific host ligands (E-cadherin on epithelial cells or N-cadherin on endothelial cells) [7]. However, the molecular network and potential cross-talk between these two mechanisms are still ill-defined. Furthermore, the contribution of hydrolytic activity to Candida invasion and interaction with host cells is largely unknown.
Secreted aspartic protease (Sap) family, consisting of 10 Saps, is the major hydrolytic enzyme produced by C. albicans. It is believed that Saps contribute both to increased endocytosis and active penetration. Previous studies showed that pepstatin A (Sap inhibitor) was able to block Candida induced endocytosis in its early stage of invasion, and reduce active penetration when combined with cytochalasin D (cytD) and butanedione monoxime (endocytosis inhibitors) [8]. Besides, significant reduction of induced endocytosis was evident in sap1-3Δ/Δ and sap4-6Δ/Δ knockout mutants, suggesting the key involvement of these Saps in this process. On the contrary, several studies showed a limited role of Saps 1-6 in Candida invasion in a reconstituted model of human epithelia as well as in a mice model [9,10]. Thus, further investigations are warranted to decipher into the functional of different Saps in Candida pathobiology.
By now, limited knowledge is available for C. albicans Sap7 for its functional significance in pathogenesis, especially in in vivo systems [11]. Here, we reported an investigation of C. albicans Sap7 in the invasion process of human oral epithelial cells.
Materials and Methods
Candida strains and culture conditions
C. albicans SC5314 was used as the wild type strain in this study for comparison. C. albicans sap7Δ/Δ mutant strain was constructed by Ura-blaster method [12] and the genotype is Δsap7::hisG/ Δsap7::hisG-URA3-hisG, which was confirmed by colony PCR with gene-specific primers. All fungal strains were prepared in liquid YPD (1% yeast extract, 2% bactopeptone, 2% D-glucose) medium on a rotary shaker at 30°C overnight. Cell concentrations were adjusted by hemocytometer counting in serum-free DMEM medium immediately prior to the experiments.
Oral epithelia cells
The OKF6/TERT2 cell line (passage 18) was a gift from J. Rheinwald’s Laboratory (Harvard University, Boston, MA) [13]. The OKF6/TERT2 cells were cultured in the humidified incubator containing 5% CO2 at 37°C. For a standard experiment, 5×104 cells/ ml of OKF6/TERT2 cells were seeded into μ-Slide 8 well plates (ibidi, Germany) and cultured for 2-3 days post-seeding until 95% confluence.
Endocytosis assay
The number of fungal cells that were associated with and/or endocytosed by the OKF6/TERT2 cells was determined using a standard differential fluorescence assay with minor modifications [14]. Briefly, 95% confluence OKF6/TERT2 cells were infected with 1×105 fungal cells in serum-free DMEM medium. After 3 hrs incubation at 37°C, the cells were gently rinsed by Hank’s Balanced Salt Solution (HBSS) twice to remove any nonadherent cells, and fixed with 3% paraformaldehyde. The adherent but not endocytosed fungal cells were stained by a polyclonal rabbit anti-Candida antibody conjugated with red fluorescent Alexa Fluor 568 for 1 h. Afterwards, the OKF6/TERT2 cells were permeabilized with 0.5% Triton X-100 in PBS for 30 min. All cell-associated fungal cells (including both adherent and endocytosed) were labelled with anti-Candida antibody conjugated with green fluorescent Alexa Fluor 488, and observed under fluorescence microscope. The number of endocytosed fungal cells was determined by subtracting the number of adherent fungal cells (red fluorescing) from the number of cell-associated fungal cells (green fluorescing). Fungal cells which were partially internalized were regarded as endocytosed cells. In each well, at least 100 fungal cells were examined.
Invasion assay
The number of fungal cells that was active penetrated to oral epithelial cells was determined as described previously [14]. Briefly, the OKF6/TERT2 cells were cultured as described above. To block the induced endocytosis, the OKF6/TERT2 cells were pre-treated with 0.5 μM cyt D for 30 min (this concentration of cyt D was found efficient to stop the rearrangement of actin cytoskeleton without affecting cell viability [8]) and co-incubated throughout the whole infection period. Thus, 1×105 fungal cells in serum-free DMEM medium were allowed to infect the OKF6/TERT2 cells for 3 h. Afterwards, nonadhesive fungal cells were gently removed by HBSS washings, the OKF6/TERT2 cells were fixed as described above. All adherent fungal cells were stained with red-fluorescent Alexa Fluor 568. After further rinsing, the OKF6/TERT2 cells were permeabilized, and associated fungal cells were stained with green-fluorescent Alexa Fluor 488 as described above. The percentage of invaded fungal cells was determined by the number of internalized cells (including partially internalized cells) by the number of all the adherent cells. At least 100 fungal cells were examined.
Damage assay
Tissue damage of the OKF6/TERT2 cells was determined by measuring the amount of released Lactate Dehydrogenase (LDH) into the surrounding medium upon invasion by C. albicans SC5314 and C. albicans sap7Δ/Δ mutant strain using CytoTox 96 nonradioactive cytotoxicity assay [15].
Statistical analysis
All experiments were performed in three separate occasions, and triplicate was performed in each occasion. The difference between the wild type C. albicans SC5314 and the C. albicans sap7Δ/Δ mutant strain was statistically assessed by Student’s t-test. A P value <0.05 was considered statistically significant.
Results and Discussion
The present study was a continuation of our ongoing research on the elucidation of the functional significance of C. albicans Saps in its pathobiology. Our previous study clearly revealed, for the first time, the potential role of C. albicans Sap9 in serum induced-hyphal formation and interaction with human oral epithelial cells [15], and laid a foundation for further investigations of ill-defined Saps, including Sap7. Previous studies showed a correlation between Sap7 and virulence using an intravenous candidiasis model [8], and was proved to be insensitive to pepstatin A [16]. Here, we investigated the functional significance of Sap7 in C. albicans invasion to human oral epithelial cells using a C. albicans sap7Δ/Δ mutant strain and compared it with the wild type counterpart.
Sap7 plays a key role in C. albicans induced endocytosis, active penetration, and cell damage in human oral epithelial cells
Induced endocytosis and active penetration are two distinct routes for microorganism’s invasion into non-phagocytic host cells such as oral epithelial cells and endothelial cells. The ability of the C. albicans sap7Δ/Δ mutant strain in induced endocytosis was found reduced in all the fields examined (Figure 1). A reduction of 52% (P<0.05) of induced endocytosis was observed in the C. albicans sap7Δ/Δ mutant strain on OKF6/TERT2 cells (Figure 2A); the number of cell-associated C. albicans sap7Δ/Δ mutant strain was 34% less than the wild type C. albicans SC5314, although the results were not statistically significance. It is envisaged that Sap7 facilitates Candida induced endocytosis via possible cellular interactions with other invasins on hyphae and/or other host cell receptors which warrant further investigations.
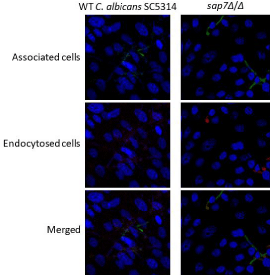
Figure 1: Fluorescence microscopic analysis of the interaction between
Candida cells and human oral epithelial cells. The endocytosed cells were
labelled as red fluorescence while the associated cells were labelled as green
fluorescence.
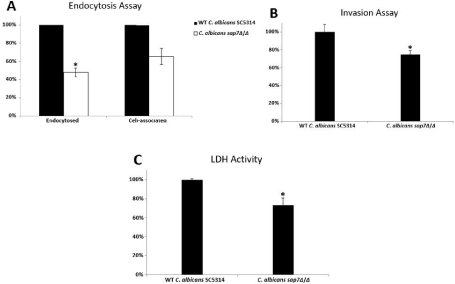
Figure 2: Analyses of the functional role of C. albicans Sap7 in (A) endocytosis; (B) invasion; and (C) LDH activity upon co-incubation with the human oral epithelial
cells (*P<0.05).
To investigate the role of Sap7 in active penetration, we coincubated the fungal cells with the human oral epithelial cells in the presence of the specific microfilament inhibitor cytD (0.5 μm) in an attempt to selectively block all induced endocytosis. The proportion of the C. albicans sap7Δ/Δ mutant strain that penetrated into the OKF6/TERT2 cells was significantly smaller than (25%; P<0.05) that of the wild type C. albicans SC5314 (Figure 2B), suggesting that Sap7 might play a key role in C. albicans active penetration in human oral epithelial cells.
We measured the amount of released LDH into co-culture supernatant to investigate quantitatively the degree of tissue damage caused by the fungal cells. The ability of the C. albicans sap7Δ/Δ mutant strain to infect the OKF6/TERT2 cells was moderately reduced by 27% (P<0.05) when compared with the wild type C. albicans SC5314, suggesting that the knockout of Sap7 gene led to a weakened invasion power to the human oral epithelial cells (Figure 2C).
Conclusion
Taken together, our present study clearly indicated that the C. albicans sap7Δ/Δ mutant strain exhibits attenuated capacity to adhering to and endocytosed by the human oral epithelial cells, and therefore suggesting that C. albicans Sap7 contributes to tissue invasion process and leads to host cell damage. As little is known about the cellular pathways/interacting molecules involved in C. albicans Sap7 pathogenesis, further investigations using proteomic study are warranted.
Funding
The present study was supported by the funding of the University of Hong Kong (No.: 201007176095).
References
- Mohr A, Simon M, Joha T, Hanses F, Salzberger B, Hitzenbichler F. Epidemiology of candidemia and impact of infectious disease consultation on survival and care. Infection 2020; 48: 275-284.
- Lim CSY, Rosli R, Seow HF, Chong PP. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. 2012; 31: 21-31.
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013; 62: 10-24.
- Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med. 2016; 34: 21-28.
- Friedman DZP, Schwartz IS. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi (Basel). 2019; 5: 67.
- Sheppard DC, Filler SG. Host cell invasion by medically important fungi. Cold Spring Harb Perspect Med. 2014; 5: a019687.
- Yang W, Yan L, Wu C, Zhao X, Tang J. Fungal invasion of epithelial cells. Microbiol Res. 2014; 169: 803-811.
- Dalle F, Wachtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010; 12: 248-271.
- Lermann U, Morschhauser J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology. 2008; 154: 3281-3295.
- Correia A, Lermann U, Teixeira L, Cerca F, Botelho S, da Costa RM, et al. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect Immun. 2010; 78: 4829-4849.
- Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008; 154: 3266-3280.
- Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005; 4: 298-309.
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000; 20: 1436-1447.
- Yang H, Tsang PCS, Pow EHN, Lam OLT, Tsang PWK. Potential role of Candida albicans secreted aspartic protease 9 in serum induced-hyphal formation and interaction with oral epithelial cells. Microb Pathog. 2020; 139: 103896.
- Tsang PWK, Fong WP, Samaranayake LP. Candida albicans orf19.3727 encodes phytase activity and is essential for human tissue damage. PLoS One. 2017; 12: e0189219.
- Aoki W, Kitahara N, Miura N, Morisaka H, Yamamoto Y, Kuroda K, et al. Candida albicans possesses Sap7 as a pepstatin A-insensitive secreted aspartic protease. PLoS One 2012; 7: e32513.