
Research Article
J Bacteriol Mycol. 2021; 8(5): 1183.
Bacterial and Fungal Co-Infections in Patients with COVID-19 Related Pneumonia: A Retrospective Cohort Study
Bedini A¹*, Menozzi M¹, Cuomo G¹, Franceschini E¹, Orlando G¹, Santoro A¹, Cozzi-Lepri A², Meschiari M¹, Carli F¹, Puzzolante C¹, Milic J¹, Del Monte M¹, Gaetano MD¹, Bacca E¹, Franceschi G¹, Dolci G¹, Busani S³, Biagioni E³, Tutone M¹, Sarti M4, Girardis M³, Guaraldi G¹ and Mussini C¹
1Department of Infectious Diseases, Azienda Ospedaliero-Universitaria di Modena “Policlinico di Modena”, Italy
2Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CRÈME), Institute for Global Health, University College London, London, UK
3Department of Anaesthesia and Intensive Care Unit, Azienda Ospedaliero-Universitaria di Modena “Policlinico di Modena”, Italy
4Clinical Microbiology Laboratory, Azienda Ospedaliero-Universitaria di Modena “Policlinico di Modena”, Italy
*Corresponding author: Andrea Bedini, Department of Infectious Diseases, Azienda Ospedaliero-Universitaria di Modena “Policlinico di Modena”, Via del pozzo 71, 41124 Modena, Italy
Received: June 04, 2021; Accepted: July 19, 2021; Published: July 26, 2021
Abstract
Background: The study analyzed risk factors for bacterial and fungal coinfection in patients with COVID-19 and the impact on mortality.
Methods: This is a single-center retrospective study conducted on 387 patients with confirmed COVID-19 pneumonia admitted to an Italian Tertiarycare hospital, between 21 February 2020 and 31 May 2020. Bacterial/fungal coinfection was determined by the presence of characteristic clinical features and positive culture results. Multivariable logistic regression was used to analyze risk factors for the development of bacterial/fungal co-infection after adjusting for demographic characteristics and comorbidities. Thirty-day survival of the patients with or without co-infections was analyzed by Kaplan- Meier method.
Results: In 53/387 (13.7%) patients with COVID-19 pneumonia, 67 episodes of bacterial/fungal co-infection occurred (14 presented >1 episode). Pneumonia was the most frequent co-infection (47.7%), followed by BSI (34.3%) and UTI (11.9%). S. aureus was responsible for 24 episodes (35.8%), E. coli for 7 (10.4%), P. aerugionsa and Enterococcus spp. for 5 episodes each (7.4%). Five (7.4%) pulmonary aspergillosis, 3 (4.4%) pneumocystosis and 5 (7.4%) invasive candidiasis were observed. Multivariable analysis showed a higher risk of infection in patients with an age >65 years (csHR 2.680; 95% CI: 1.254-5.727; p=0.054), with cancer (csHR 5.243; 95% CI: 1.173-23.423; p=0.030), with a LOS >10 days (csHR 12.507; 95% CI: 2.659 - 58.830; p=0.001), early (within 48h) admitted in ICU (csHR 11.766; 95% CI: 4.353-31.804; p<0.001), and with a SOFA score >5 (csHR 3.397; 95% CI: 1.091-10.581; p=0.035). Estimated cumulative risk of developing at least 1 bacterial/fungal co-infection episode was of 15% and 27% after 15 and 30 days from admission, respectively. Kaplan-Meier estimated a higher cumulative probability of death in patients with bacterial/fungal co-infection (log-rank=0.031). Thirty-day mortality rate of patients with pneumonia was 38.7%, higher than those with BSI (30.4%).
Conclusions: Bacterial and fungal infections are a serious complication affecting the survival of patients with COVID-19- related pneumonia. Some issues need to be investigated, such as the best empirical antibiotic therapy and the need for possible antifungal prophylaxis.
Keywords: COVID-19; SARS-CoV-2 pneumonia; Bacterial co-infection; Aspergillosis; CAPA; Fungal co-infections
Abbreviations
COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2; ARDS: Acute Respiratory Distress Syndrome; BSI: Bloodstream Infections; ICU: Intensive Care Unit; LOS: Length of Stay; SaO2: Oxygen Saturation; UTI: Urinary Tract Infection; PCR: Polymerase Chain Reaction; ABSSIs: Acute Bacterial Skin and Soft Tissue Infections; CRP: C-Reactive Protein; SOFA: Sequential Organ Failure Assessment; NIV: Non-Invasive Ventilation; MDR: Multi-Drug Resistant; ESBL+: Extended-Spectrum Beta-Lactamase Producer; GNB: Gram-Negative Bacilli; CR: Carbapenem-Resistant; VRE: Vancomycin- Resistant Enterococcus; MR: Methicillin-Resistant; MRSA: Methicillin-Resistant Staphylococcus aureus; SIMIT: Italian Society of Infectious Diseases; csHR: cause-specific Hazard Ratio; IQR: Interquartile Range; CI: Confidence Interval
Background
The outbreak of COVID-19, due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), began in Wuhan, Hubei Province, China, and has subsequently spread all over the world. Up to 30% of patients admitted to hospital with COVID-19 need admission to Intensive Care Units (ICUs) to receive ventilation assistance because they develop Acute Respiratory Distress Syndrome (ARDS) [1-3].
Early reports from China suggested that co-infection with other respiratory pathogens was rare. If this were the case, patient’s positive for other pathogens might be assumed unlikely to have SARSCoV- 2. The Centers for Disease Control and Prevention endorsed testing for other respiratory pathogens, suggesting that evidence of another infection could aid the evaluation of patients with potential COVID-19 in the absence of widely available rapid testing for SARSCoV- 2 [4].
The prevalence of co-infection varied among COVID-19 patients, ranging from 0% to 50% among non-survivors. Chinese publications reported at least 10% of co-infection during COVID-19 in patients hospitalized in ICU for ARDS, among them Aspergillus infections [1,2]. Besides, the incidence of Invasive Pulmonary Aspergillosis (IPA) in ICU patients admitted for severe influenza A and B is high, reaching 19% versus 5% in patients with severe pneumonia other than flu [3].
A recent Spanish retrospective cohort study [5] showed that bacterial or fungal co-infection at COVID-19 diagnosis is uncommon (3.1%) and that few patients developed co-infections during hospitalization (5.1%). Overall, patients with co-infections resulted to have worse outcomes.
The prevalence of laboratory confirmed bacterial superinfection in critically ill COVID-19 patients admitted in ICU is around 14% (95% confidence interval 5-26%) according to a recent metaanalysis [6]. However, in most included studies there was no distinction between early and late infections. This is a key point in deciding whether antibiotic prescribing in the critical ill patients with COVID-19 is truly helpful in preventing infection or is a risk factor for the development of antimicrobial resistance. Many studies of hospitalized patients with COVID-19 note the empiric use of antibiotics in a majority of patients [7-9]. However, there is evidence that the inflammatory serological markers that are usually associated with bacterial infection, such as raised procalcitonin and C-reactive protein, may appear in patients with COVID-19 without a corresponding bacterial co-infection occurring [10,11].
The aim of the study was to describe the bacterial and fungal co-infections in the patients admitted for COVID-19 pneumonia, evaluating the risk factors for co-infection and the impact of the coinfections on the 30-day mortality rate.
Methods
This retrospective cohort study was performed at the “Policlinico di Modena” Hospital (Italy), a 550-bed University Centre that provides broad and specialized medical, surgical, and intensive care for an urban population of 150,000 adults. All patients (≥18 years) admitted for COVID-19 pneumonia from 21 February to 1 May 2020 were included. Diagnostic criteria for COVID-19 pneumonia comprised the presence of any of the following respiratory symptoms, including sore throat, congestion, cough, dyspnoea, new loss of taste and/or smell, as well as uni- or bilateral interstitial infiltrates in the chest X-ray. The diagnosis was confirmed by real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR) testing performed on nasopharyngeal throat swab specimens.
Patients’ demographic, epidemiologic and clinical data were collected in an electronic patient chart.
All patients received treatment according to the Italian Society of Infectious Diseases’ Guidelines (SIMIT) recommendations [12] including oxygen supply to target SaO2 >90%, hydroxychloroquine with or without azithromycin, and low molecular weight heparin. Lopinavir/ritonavir or darunavir/cobicistat was also used up to 18 March, when a clinical trial on the former did not show any benefit of protease inhibitors against the standard of care [13].
The aim of the study was to describe the bacterial and fungal co-infections in the patients admitted for COVID-19 pneumonia, evaluating the risk factors for co-infection and the impact on the survival.
Definitions
Bloodstream Infection (BSI) was defined in presence of at least one positive blood culture for bacteria or fungi; for coagulasenegative staphylococci and other common skin contaminants, at least two consecutive blood cultures positive for the same pathogen were necessary to define BSI. Bacterial and fungal pneumonia was defined by the appearance of new consolidation or interstitial lung infiltrates associated to respiratory signs (coughing, dyspnoea, production of purulent exudate) and the identification of the responsible microorganism through culture or real-time Polymerase Chain Reaction (PCR) test from sputum and/or lower respiratory tract specimens, or the detection of a urinary antigen for Streptococcus pneumonia or Legionella species. Urinary Tract Infection (UTI) was defined as the appearance of fever with or without urinary signs of infection, with the isolation of a microorganism from the urine culture test. If diagnosis was at onset or within the first 48h of COVID-19 hospital admission, these infections were defined as community-acquired co-infections. If diagnosis occurred ≥48h of admission for COVID-19, these infections were defined as hospitalacquired co-infections.
Data collection
The following data were collected from the patients’ electronic medical records: age in years; gender; Sequential Organ Failure Assessment (SOFA) score at the admission in the hospital [14], Length of Stay (LOS), admission in Intensive Care Unit (ICU), the need for Non-Invasive (NIV) and invasive mechanical ventilation (intubation) for severe respiratory failure, and the possible treatment with antiinflammatory drugs (tocilizumab, anakinra and steroids). The antiinflammatory therapy was administered in the following dosages: tocilizumab, intravenously or subcutaneously, at 8mg/kg, possibly repeated after 12 hours if inflammatory activity persists; anakinra subcutaneously at the dose of at 1-2 mg/kg/24 hours, increasing by 0.5-1 mg/kg/die until the control of active inflammation, never exceeding the 8mg/kg/die; and intravenous methylprednisolone at 1mg/kg/24 hours.
Statistical analyses
Demographic and clinical characteristics of the patients were summarized with number and percentages for categorical variables, and with median and Interquartile Range (IQR) for continuous variables. The possible association of demographic and clinical variables with development of bacterial/fungal infections was first tested in univariable Cox regression models for estimating the unadjusted cause-specific Hazard Ratio (csHR) for development of infection. Then, variables potentially associated with infections in univariable comparisons (p ≤0.09) were included in a multivariable Cox regression model for calculating the adjusted csHR for development of co-infection.
In the analysis of the risk factors for bacterial/fungal co-infection, cases where the infection had occurred within the first 48 hours of admission were excluded. The following risk factors for infection were assessed: gender, age >65 years, LOS >10 days, SOFA Score >5, chronic renal failure, cancer, leucocytes>10x10³/μL, C-reactive protein >10 mg/dl, beginning of symptoms >10 days, early admission in ICU (within 48 hours of admission), and early need of noninvasive ventilation (within 48 hours of admission). The cumulative risk of in-hospital acquired co-infections was calculated using the Aalen-Johansen method, considering the first occurring co-infection as the event of interest, death and discharge from the hospital as competing events. Chi-square test was used to evaluate the impact of empirical antibiotic therapy on the development of co- infection by Multi-Drug Resistant (MDR) microorganisms: Methicillin-Resistant Staphylococcus spp. (MR-S), Extended-Spectrum Beta-lactamase producer bacilli Gram-negative (ESBL+GNB), carbapenemresistant Gram-negative bacilli (CR-GNB), Vancomycin-resistant Enterococcus species (VRE). ESBL+, CR, MRSA, VRE). Thirty-day survival of the patients with or without co-infections was described with the Kaplan-Meier method, with death as the event of interest and discharge from hospital as right censoring events.
Statistical analyses were performed using SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Between 21 February and 1 May 2020, 387 patients with COVID-19 pneumonia were admitted to the University Hospital of Modena, Italy. In all patients, the diagnosis was confirmed by PCR method on nasopharyngeal swab. The study population was represented by 267 males (69.0%) and the median age was 64 years (IQR 24-94 years). Median LOS was 12 days (IQR: 1-89 days) and 101 (26.1%) patients were admitted to ICU for at least 24 hours. Three hundred and fifteen (81.4%) patients received empirical antibiotic therapy on admission, represented in 84.1% (265 patients) by thirdgeneration cephalosporin. In the Table 1, clinical and demographic characteristic of the study population are summarized. One hundred and fifty-two (39.2%) patients had severe respiratory failure and needed mechanical ventilation: 76 (19.6%) patients required also an invasive mechanical ventilation, while 76 (19.6%) underwent noninvasive mechanical ventilation, only. One hundred and seventy-one (44.2%) patients received tocilizumab therapy (8 mg/kg/12 hours for a total of 2 administrations administered intravenously in 99 patients, and 2 doses of 162 mg subcutaneously, 1 per tight, in 72 patients). Fifteen (3.9%) patients received anakinra; all these patients were in ICU at the moment of the administration. One hundred and seven (27.6%) patients received also glucocorticoid therapy (methylprednisolone 1 mg/kg/24h).
Variable
N = 387
Male Gender
267 (69.0%)
Age in years, Median (IQR)
64 (range 24-94)
Length of stay, Median (IQR)
12 (1-89)
Admission in ICU (for >24 hours)
101 (26.1%)
Invasive Mechanical Ventilation
76 (19.6%)
Non-invasive Mechanical Ventilation, only
76 (19.6%)
Empirical antibiotic therapy on admission
315 (81%)
Chronic renal failure (N.A*: 368)
50 (13.5%)
Cancer
14 (3.6%)
Leucocytes (N/mL), Median (IQR)
6,215 (range 147-61,340)
Reactive C protein (mg/dl), Median (IQR)
6.5 (range 0.2-36.8)
Tocilizumab Treatment
171 (44.2%)
Anakinra Treatment
15 (3.9%)
Glucocorticoids
107 (27.6%)
ICU: Intensive Care Unit; NIV: Non-Invasive Ventilation; IQR: Interquartile Range; N.A: Number Available.
Table 1: Clinical and demographic characteristic of the patients admitted to the University Hospital of Modena for COVID-19 pneumonia (February 21, 2020-May 1, 2020).
The mortality rate after 30 days of hospitalization was 19.9 % (77 patients), while overall mortality, calculated at the time of discharge, was 21.4% (83 patients).
Bacterial and fungal co-infections
Among the 387 patients admitted for SARS-CoV-2 pneumonia, 67 (17.3%) episodes of bacterial or fungal co-infection were observed in 53 patients: 32 (8.2%) pneumonia, 23 (5.9%) BSIs, 8 (2.0%) UTIs, and 3 (0.7%) other co-infections (1 ABSSSIs, 1 peritonitis and 1 dental abscess). The 49.2% (33/67) of the bacterial and fungal infections occurred in ICU: 13/23 (56.5%) of BSIs and 25/32 of pneumonia (78.1%). Of these 53 patients, 45 (84.9%) acquired co-infection 48 hours after admission (Hospital-Acquired Infection, HAI) with a median time of 9 days (IQR 3-33 days) from admission.
Microbiological characteristics and resistance rates are summarized in Table 2. Co-infections were caused by Gram positive microorganisms in 55.2% of cases (n=37), bacilli Gram negative in 26.8% (n=18) and fungi in 17.9% (n=12). The most frequently microorganism isolated was S. aureus (35.8%), followed by E. coli (10.4%), Coagulase Negative Staphylococcus (8.9%), P. aeruginosa (7.4%) and Enterococcus spp. (7.4%). S. aureus methicillinresistance rate was 54.1%, while the prevalence of ESBL-producers among Enterobacteriacae resulted 46.1%. Only one strain resulted carbapenem-resistant (CR), a P. aeruginosa isolate.
Pneumonia (n=32)
BSI (n=23)
UTI (n=8)
Others* (n=3)
Total (n=67)
S. aureus (MR)
14 (8)
9 (4)
0 (0)
1 (1)
24 (13)
Coagulase Neg Staphylococcus (MR)
0 (0)
5 (5)
0 (0)
0 (0)
6 (6)
Escherichia coli (ESBL+)
1 (0)
1 (0)
5 (3)
0 (0)
7 (3)
Klebsiella pneumoniae (ESBL+)
3 (3)
1 (0)
0 (0)
0 (0)
4 (3)
Enterobacter cloacae (ESBL+)
2 (0)
0 (0)
0 (0)
0 (0)
2 (0)
Pseudomonas aeruginosa (CR)
3 (1)
0 (0)
2 (0)
0 (0)
5 (1)
Enterococcus spp. (VRE+)
1 (0)
2 (1)
1 (0)
1 (1)
5 (2)
Streptococcus pneumoniae
0
2
0
0
2
Candida spp.
0
3
0
1
4
Aspergillus spp.
5
0
0
0
5
Pneumocystis jirovecii
3
0
0
0
3
BSI: Bloodstream Infection; UTI: Urinary Tract Infection; MR: Methicillin-Resistant; ESBL+: Extended-Spectrum Beta- Lactamase Producer; CR: Carbapenem-Resistant. *Others: 3 Acute bacterial skin and soft tissue infection, 1 bacterial spontaneous peritonitis and 1 dental abscess.
Table 2: Microbiological characteristics and resistance rates of the 67 episodes of bacterial/fungal co-infections occurred in patients admitted for COVID-19 pneumonia.
The following fungal infections were also observed: 5 (7.4%) pulmonary aspergillosis, 4 (5.9%) infections by Candida spp. (3 candidemias and 1 subcutaneous abscess), and 3 (4.4%) Pneumocystis jirovecii pneumonia.
Of the 45 patients who developed HAI, 37 (82.2%) received empiric antibiotic therapy at admission. We evaluated the impact of antibiotic therapy on the development of co-infection by MDR microorganisms (especially by MR-S, ESBL+GNB, CR- GNB, VRE). Chi square analysis showed that patients who received empiric antibiotic therapy had a higher risk of developing an MDR-bacteria co-infection (19/37, 51.3%) than those who did not receive antibiotics (1/8, 12.5%) (p=0.045).
Univariate analysis of the risk factors for acquiring nosocomial bacterial or fungal co-infection showed a significant association of the following factors: age >65 years (p=0.017), cancer (0.056), leucocytes >10x10³/μL (p=0.016), length of stay>10 days (p<0.001), early admission in ICU (p<0.0001), and early need of non-invasive ventilation (p<0.0001). Multivariate analysis confirmed a higher risk of infection in patients aged >65 years (csHR 2.680; 95% CI: 1.254 - 5.727; p=0.054), with cancer (csHR 5.243; 95% CI: 1.173-23.423; p=0.030), with a LOS >10 days (csHR 12.507; 95% CI: 2.659 -58.830; p=0.001), early admitted in ICU (csHR 11.766; 95% CI: 4.353-31.804; p<0.001), and with a SOFA score >5 (csHR 3.397; 95% CI: 1.091 - 10.581; p=0.035) (Table 3).
Unadjusted cause-specific HR (95%CI)
P-value
Adjusted cause-specific HR (95%CI)
P-value
Male Gender
1.121 (0.566-2.221)
0.744
1.386 (0.545-3.526)
0.493
Age >65 years
2.222 (1.154-4.279)
0.017
2.307 (0.985-5.403)
0.054
Chronic renal failure
1.005 (0.401-2.516)
0.992
-
-
Cancer
3.239 (0.972-10.798)
0.056
5.243 (1.173-23.423)
0.03
Leucocytes >10x103/mL
2.570 (1.191-5.547)
0.016
1.053 (0.403-2.751)
0.915
Reactive C protein >10mg/dL
1.189 (0.608-2.325)
0.612
-
-
Time from initial symptoms and admission >10 days
0.751 (0.366-1.539)
0.434
-
-
LOS >10 days
12.453 (3.787-40.950)
<0.001
12.507 (2.659-58.830)
0.001
SOFA Score >5
1.987 (0.918-4.300)
0.081
3.397 (1.091-10.581)
0.035
Early ICU admission (=48h)
10.871 (5.511-21.442)
<0.0001
11.766 (4.353-31.804)
<0.001
Early need of NIV (=48h)
3.985 (2.059-7.710)
<0.0001
1.665 (0.673-4.123)
0.27
LOS: Length of Stay; SOFA: Sepsis-Related Organ Failure Assessment; ICU: Intensive Care Unit; NIV: Non-Invasive Ventilation.
Table 3: Univariate and multivariate analysis of risk factors for the development of nosocomial bacterial/fungal co- infections in patients with COVID-19 pneumonia.
Estimated cumulative risk of developing at least 1 bacterial/fungal co-infection episode was of 15% and 27% after 15 and 30 days at risk, respectively (Figure 1).
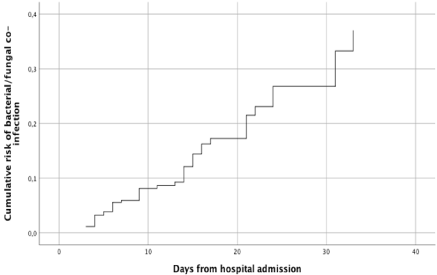
Figure 1: Cumulative risk of bacterial/fungal co-infections in patients with
COVID-19 pneumonia.
Legend: Cumulative risk of bacterial/fungal co-infection in patients with
COVID-19 at different lengths of stay, with the first occurring hospital-acquired
infection as the event of interest and death and discharge from the hospital
as competing events.
Chi square analysis showed that patients who received empiric antibiotic therapy (n=37) had a higher risk of developing an MDRbacteria co-infection (51.3%) than those who did not receive it (12.5%, p=0.045).
In Figure 2, Kaplan-Meier estimates the cumulative probability of death based on the presence or absence of bacterial/fungal coinfection. As can be seen from the curve, the risk of death becomes significant after 13 days of hospitalization (log-rank=0.031).
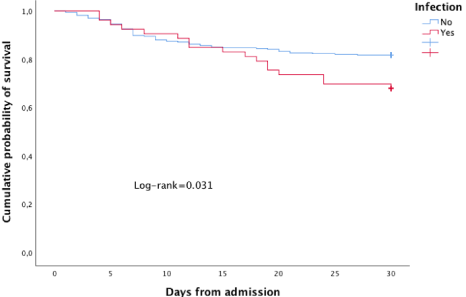
Figure 2: Kaplan-Meier estimates of the cumulative probability of death at
30-day from admission by bacterial/fungal co-infection.
Thirty-day mortality rate of patients with pneumonia was 38.7%, higher than those with BSI (30.4%). Among patients with pneumonia, the highest 30-day mortality rate was observed in patients with pneumonia by Pneumocystis jirovecii (66.6%) and Aspergillus app. (40.0%), while in those with S. aureus pneumonia it was 21.4%.
Discussion
The exact incidence of bacterial and fungal co-infections in COVID-19 is unknown, and while there are anecdotal reports of documented bacterial superinfections, the incidence seems to be much lower than in other viral diseases as severe influenza [15,16]. In our population, the prevalence of bacterial and fungal co-infections was 17.3%. These findings are in line with the results (1-12 %) reported in other epidemiological reports from China and the US [1,3,17]. Among 16,654 Italian patients who died of COVID-19 during the first wave of pandemic, bacterial and fungal superinfections were reported in 11.0% of cases [18]. In another Italian study [19], a very high prevalence of BSI was found in patients admitted in ICU with COVID-19 (45 episodes/71 patients, 63.3%), and a 24-day mortality rate of 25.0% was observed. In our study, the episodes of BSI occurred in ICU were much less frequent (13/101, 12.8%).
In our population, most of the cases were caused by Gram positive bacteria (55.2%), and only 26.8% by Gram negative bacilli. In addition, the rate of methicillin resistance was high (51.4%9). These two data can be explained by the fact that 81.4% of patients admitted to our hospital with COVID-19 received empirical antibiotic therapy and that in the 84.1% of the cases it was represented by a thirdgeneration cephalosporin (most active on Gram negative bacilli). The rationale for antibiotic treatment in patients with COVID-19 was based on the experience with bacterial superinfection in influenza, where most studies reported initial co-infection or secondary bacterial pneumonia (11-35 % of cases) in hospitalized patients [20]. Generally, most patients admitted with COVID-19 pneumonia had fever, hypoxic respiratory failure, and increased of blood value of C Reactive Protein (CRP) for increased inflammatory activity. These signs and alterations worried clinicians who preferred to prescribe empirical/pre-emptive antibiotic therapy, at least during the first 3-5 days of hospitalization. A recent review [21] showed that 88.3% of COVID-infected patients (476/539) were treated with broadspectrum antibiotics including third-generation cephalosporins, quinolones, and carbapenems.
In our population, we observed a higher prevalence of infection caused by MDR microorganisms in patients who had received empirical antibiotic therapy. Fortunately, only 1 case of GNB CR was isolated (1 P. aeruginosa strain). This data is very significant, considering the high prevalence rates of GNB CR infection in Italian hospitals. We think this may be the result of an antimicrobial stewardship program put in place in our hospital starting in 2014 [22,23]. This observation raises an interesting question as to what considerations should be made before prescribing empirical antibiotic therapy in COVID-19 infected patients, assessing its advantages and disadvantages. Bio-humoral markers such as procalcitonin should be used by clinicians to discriminate when to start empirical antibiotic therapy, or when to stop it, in patients hospitalized for severe COVID-19 disease.
Based on our findings, the idea of prescribing all patients with a higher-spectrum antibiotic therapy (e.g. anti-MRSA antibiotic therapy) does not seem justified, as we believe that it was the effect of antibiotic therapy that created the selection of strains resistant to methicillin. The use of anti-MRSA antibiotics could select even more resistant microorganisms (i.e. vancomycin-resistant Enterococcus faecium or Coagulase-negative Staphylococcus resistant to linezolid). On the other hand, in centers where an antimicrobial stewardship program was active, no increase in MDR microorganisms BSI was observed during the first wave of the COVID-19 pandemic [24]. In the study of Guisado-Gil AB et al. the frequency of empirical treatment of patients with COVID-19 infections was quite lower (33.7%), resulting in a lower incidence of invasive MDR microorganisms BSI and a lower incidence of candidemia. Thus, when the probability of a bacterial infection is low antibiotics should not be prescribed and any empiric antibiotic therapy should be discontinued immediately. Antibiotics should be reserved for patients with the most severe respiratory presentations [25,26]. In our study, multivariate analysis of factors related to an increased risk of bacterial infection after hospitalization showed that patients with cancer, with a hospital stay >10 days, with a SOFA Score >5, and who were admitted early to the ICU or who required NIV rapidly had an increased risk of bacterial superinfection. In these patients, the addition of antibiotic therapy could be considered if fever persisted or the clinical respiratory picture worsened. Finally, we observed that estimated cumulative risk of developing at least 1 episode of superinfection was of 15% and 27% after 15 and 30 days at risk, respectively.
Equally, attention should be paid when assessing cases of possible invasive fungal infection, particularly cases of Invasive Pulmonary Aspergillosis (IPA) and P. jirovecii pneumonia. Several studies had reported the occurrence of COVID-19 associated with IPA. The largest series was shown by Zhu et al. [27] in Jiangsu Province, China (January 22-February 2, 2020) in which 23.3% (60/243) COVID-19 patients had co-infection with Aspergillus. Several studies in Europe [28-30] reported different incidence rates of IPA among COVID-19 patients admitted in ICU: 19.6% in Netherland, 20.6% in Belgium and 33.3% in France. In our population, 5 cases (1.2%) of IPA (3 probable and 2 possible) and 3 (0.7%) cases of P. jirovecii pneumonia have been observed. All the fungal infections occurred in patients admitted in ICU and considering only these setting (n=101), the incidence of IPA and P. jirovecii pneumonia was very low (4.9 and 2.9%, respectively). The data showed that the incidence of pulmonary fungal infections in our population is lower than other studies. The diagnosis of aspergillosis and pneumocystosis is not easy in patients with COVID-19 pneumonia because one of the diagnostic criteria (the radiological one) is of scarce use because it can be confused with the alterations due to COVID-19. Moreover, being ubiquitous and opportunistic fungi, it is not uncommon to find them in the respiratory material of hospitalized and intubated patients. In addition, broncho-alveolar lavage is an invasive procedure highly at risk of infection for clinicians when performed in a patient with COVID-19 and is therefore rarely performed. For these reasons and in light of low sensitivity of serum galactomannan, more sensitive blood tests should be performed, e. g. PCR for Aspergillus, β-Dglucan, and the Aspergillus-specific lateral flow device and lateral flow assay.
Invasive candidiasis was also rare in our population (3 cases of candidemia and 1 case of subcutaneous abscess, 1.0%). Based on our data, and in consideration of the possible side effects of antifungal drugs as voriconazole, we do not believe that the introduction of antifungal prophylaxis in patients with COVID-19 related pneumonia is justified.
Conclusions
In conclusion, bacterial and fungal infections represent a serious complication that can slow down the healing process of the patient with COVID-19 related pneumonia and can sometimes cause death. Some issues need to be investigated, such as the best empirical antibiotic therapy, the need for possible anti-fungal or anti-viral prophylaxis, and what are the best methods for the diagnosis of certain co-infections associated with COVID-19 pneumonia (e.g. pulmonary aspergillosis). Controlled randomised trials could help shed light on these outstanding issues.
Declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethical Committee of Emilia Romagna (protocol number: AOU 0018046/20).
Consent to participate
Patients provided verbal, not written, informed consent because of isolation precautions. This procedure was approved by the Regional Ethical Committee of Emilia Romagna (protocol number: AOU 0018046/20).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author’s Contributions
All authors read and approved the final version of the manuscript to be submitted for publication. AB and ACL performed the statistical analysis. AB, CM and MMes have contributed to drafting and revision of the manuscript. All authors read and approved the final version of the manuscript to be submitted for publication.
Acknowledgments
We thank Barbara Beghetto, Giulia Nardini, and Enrica Roncaglia at the office of Clinical Protocols and Data Management in Modena, Rossella Fogliani at the office of Information and Communication Technologies of the Policlinico di Modena, and Nilla Viani, Marianna Rivasi, Lisa Daya, Laura Cancian, and Cinzia Barberini from the Pharmacy of Modena.
The authors would like to thank the Modena COVID-19 Working Group.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323: 1061-1069.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395: 507-513.
- Evaluating and Testing Persons for Coronavirus Disease. 2019 (COVID-19). Centers for Disease Control and Prevention. 2020.
- Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021; 27: 83- 88.
- Lanbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systemic review and meta-analysis. J infect. 2020: 81: 266-275.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
- Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020; 71: 769- 777.
- Du RH, Liu LM, Yin W, Wang W, Guan LL, Yuan ML. Hospitalization and Critical Care of 109 Decedents with COVID-19 Pneumonia in Wuhan. China. 2020.
- Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y. Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing. J Med Virol. 2020.
- Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020; 55: 1169-1174.
- National Society of Infectious Diseases’ (SIMIT) Guidelines for the management of COVID-19 infection.
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid - 19. N Engl J Med. 2020: 1-13.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22: 707-710.
- Bhatraju P. Covid-19 in critically ill patients in the Seattle region. New Engl J Med. 2020.
- Rawson TM. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020: ciaa530.
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020.
- Caratteristiche dei pazienti deceduti positivi a COVID-19 in Italia. 2020.
- Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020; e13319.
- Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, et al. The frequency of influenza and bacterial co-infection: a systematic review and meta-analysis. Influenza Other Respir Virus. 2016; 10: 394-403.
- Fattorini L, Creti R, Palma C, et al. Bacterial coinfections in COVID-19: an underestimated adversary. Ann Ist Super Sanità. 2020; 56: 359-364.
- Bedini A, Meschiari M, Franceschini E, Mussini C. 1979.Five-year impact of an antimicrobial stewardship program on nosocomial candidemia: an interrupted time-series analysis study. Open Forum Infect Dis. 2019; 6: S662.
- Meschiari M, Lopez-Lozano JM, Beyaert A, Nebot C, Orlando G, Bedini A, et al. 2443. Impact of antimicrobial stewardship on the incidence of carbapenem-resistant Pseudomonas aeruginosa: a nonlinear time-series analysis approach to identify carbapenem thresholds. Open Forum Infect Dis. 2019; 6: S844-845.
- Guisado-Gil AB, Infante-Domínguez C, Peñalva G, Praena J, et al. Impact of the COVID-19 Pandemic on Antimicrobial Consumption and Hospital- Acquired Candidemia and Multidrug-Resistant Bloodstream Infections. Antibiotics (Basel). 2020; 9: 816.
- Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary care setting. Clinical Microbiology and Infection. 2020; 26: 1395-1399.
- Huttner BD, Catho G, Pano-Pardo JR, Pulcini C, Schouten J. COVID-19: don’t neglect antimicrobial stew- ardship principles! Clin Microbiol Infect. 2020; 26: 808-810.
- Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020; 285: 198005.
- van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020; 202: 132-135.
- Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020; 10: 71.
- Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020; 8: e48-e49.