
Research Article
J Bacteriol Mycol. 2022; 9(1): 1194.
Association of Acinetobacter baumannii with Soft Rot Disease of Carrot in India
Chandrashekar BS¹, PrasannaKumar MK¹*, Buela Parivallal P¹, Pramesh D², Swathi SP¹, Sahana NB¹, Mahesh HB³ and Puneeth ME¹
1Department of Plant Pathology, University of Agricultural Sciences, Bangalore, India
2Rice Pathology Laboratory, All India Coordinated Rice Improvement Programme, Gangavathi, University of Agricultural Sciences, Raichur, India
3Department of Genetics and Plant Breeding, College of Agriculture, V. C. Farm, Mandya, India
*Corresponding author: MK Prasanna Kumar, Department of Plant Pathology, University of Agricultural Sciences, Bangalore, India
Received: December 24, 2021; Accepted: January 31, 2022; Published: February 07, 2022
Abstract
Soft rot disease of carrots is an important limiting factor of carrot production. In this study, carrot roots showing typical soft rot symptoms were identified in the fields, and diseased and healthy root samples were collected for pathogen identification. The pathogen was isolated using an enriched bell pepper method. The bell pepper developed a water-soaked lesion around the pricking region when it was pricked after stabbing the diseased root whereas, no symptoms were produced when bell pepper was pricked after stabbing a healthy carrot root. From samples of the infected roots, circular, whitish, smooth, mucoid, round, convex, and medium-sized colonies were formed on the nutrient agar medium and were morphologically identified as Acinetobacter spp. Pure culture for four isolates was obtained, and one of the isolates (AB1) was further subjected to 16S rDNA sequencing. The BLAST analysis of the 16S rDNA confirmed the identity of AB1 as Acinetobacter baumannii. Pathogenicity test using whole-root assay and slice assay proved AB1 as pathogenic on carrot by producing water-soaked lesion, maceration, and rotting symptoms, whereas water inoculated roots remain healthy. The rotting symptoms on the artificially diseased carrot roots were similar to those caused by Pectobacterium caratovorum and Klebsiella variicola on the carrot. Based on the colony morphology, biochemical tests, and 16S rDNA sequence identity followed by pathogenicity assays, it is evident that A. baumannii causes soft rot disease in carrots. This report is essential for developing specific diagnostics and management against this newly emerging bacterial pathogen of carrot.
Keywords: Soft rot; Acinetobacter baumannii; 16S rDNA; Water-soaked lesion
Introduction
Cross-kingdom pathogens, and their potential plant reservoirs, have important implications for the emergence of infectious human/ plant diseases. However, the directionality of host association (plant to human or human to plant) is difficult to determine in cross-kingdom pathogenicity [1]. Surprisingly, some of these plant pathogens cause disease in humans and are frequently isolated from human infections in the nosocomial environment. Despite this specialization in plants, species of Pantoea have also been discovered to be pathogenic to humans. Now classified as an opportunistic human pathogen, P. agglomerans has been reported to be associated with septicaemia in humans [2]. Interestingly, Burkholderia cepacia, which causes onion rot, can also cause life-threatening pulmonary infections in humans [3]. Several recent studies have reported that these human-pathogenic bacterial species are also capable of colonizing and causing disease in many plant hosts [4]. Notably, many of these studies have been conducted under laboratory conditions, providing evidence for the phytopathogenic potentials of these cross-kingdom bacterial pathogens; but, the incidence of plant disease caused by many of these human pathogens in the natural environment remains unknown. Similarly, Enterobacter cloacae have evolved to colonize the human host, and it has also been identified as the causal agent of grey kernel disease of macadamia plants. The onset of grey kernel disease affects not only the quality of the kernels produced by the tree but results in grey discolouration and a foul smell [5]. E. cloacae also causes bacterial soft rot disease in dragon fruit [6], and bacterial leaf rots in Odontioda orchids [7]. Bacteria that cause disease in both plants and animals may have genes required to infect both hosts. For example, Pseudomonas aeruginosa causes persistent lung infections in humans also can infect plants and other hosts [8].
Bacterial soft rots are a group of diseases that cause more crop loss worldwide than any other bacterial disease. Bacterial soft rots damage succulent plant parts such as fruits, tubers, stems, and bulbs of plants in nearly every plant family [9]. Soft rot bacteria degrade pectate molecules that bind plant cells together, causing plant structure to fall apart eventually. Soft rots commonly affect vegetables such as potato, carrot, tomato, cucurbits (e.g., cucumbers, melons, squash, pumpkins), and cruciferous crops (e.g., cabbage, cauliflower, Boy Choy) [10-13]. These diseases can occur on crops in the field and on harvested crops in storage. Rot can occur over a wide temperature range, with the worst decay between 21°C and 80°C, particularly when oxygen is limited [14].
Carrot (Daucus carota subsp. sativus) is an important vegetable in India. Soft rot is a serious disease of carrots in the field that causes total loss, and rotting can also be observed in the storage. This disease is caused by Erwinia carotovora [15] and Pectobacterium carotovorum [9]. Recently, Klebsiella variicola has also been reported to cause rotting disease in carrots in India [16]. Our initial studies on soft rot infected carrot samples indicated negative for the presence of E. caratovora, P. carotovorum and K. variicola, Pseudomonas viridiflava, and P. marginalis pathogens, and the isolated bacteria was shown similarities with the Acinetobacter spp. Considering the above facts, the present study was undertaken to identify and characterize the bacterial pathogens associated with the soft rot disease of carrots. We identified an isolate of A. baumannii as the causal organism of soft rot in carrots.
Acinetobacter baumannii is a ubiquitous bacterium that exists under a wide variety of environmental conditions [17] and is found as a food contaminant [18]. A. baumannii has been reported to be associated with hospital-acquired nosocomial infections in humans [19]. A. baumannii is an opportunistic organism and showed high resistance to carbapenem throughout Asia and America [20] Pneumonia, bloodstream infections, urinal tract infections, and meningitis are the most common clinical manifestations [21]. In plants, A. baumannii has been in diverse association forms from endophytic beneficial to the disease-causing pathogen. In chilli and pearl millet, A. baumannii has been reported as beneficial to plants by producing plant hormones [19,22]. Multidrug-resistant strains of A. baumannii have also been frequently isolated from crop fields [23]. Most importantly, A. baumannii has been reported as a plant pathogen causing the top rot phase of the sugarcane red stripe disease in India [24] and dieback disease of mango in Pakistan [25]. A study also indicated the competitive nature of A. baumannii in the Ralstonia infected tomato plants for in-planta multiplication [26].
In this study, we report the isolation, identification, and characterization of a cross-kingdom infecting bacteria A. baumannii associated with the soft rot disease of carrot in India. One of the strains, AB1, was taxonomically identified through sequencing 16S rDNA, followed by biochemical and molecular characterization.
Materials and Methods
Collection of diseased samples and pathogen isolation
The carrot sample exhibiting water-soaked lesions, rotting of taproot (Figure 1) were collected from Chintamani, Karnataka, India (N 130 24' 5.4'' E0 780 03' 20.1'') during 2020. About six samples were collected along with two healthy roots. Both healthy and diseased samples were pre-cleaned in the field and brought to the laboratory for further analysis. As the rotting phase of the disease is associated with many saprophytes, an enrichment technique was followed using a healthy bell pepper. Healthy and green bell pepper was washed with running tap water, then surface sterilized with 0.5% sodium hypochlorite for 1min, followed by a wash with sterile distilled water (SDW) and air-dried. A sterile toothpick was stubbed into the diseased tissue of carrot and then stubbed into the surface-sterilized bell pepper. Inoculated peppers were then placed in a plastic bag, and the moistened wet cotton was then incubated at 30°C for 24-48 hours. The diseased/symptomatic tissue around the toothpick pricking bell pepper was aseptically removed and surface sterilized with 0.5% sodium hypochlorite solution for a few seconds and then washed with SDW. The portion of the infected region was macerated in a sterile saline solution (0.85%) using a sterile pestle and mortar under aseptic conditions. The resulting suspension was left undisturbed for a few minutes for the bacterium to release from the tissue. This suspension was then streaked on Nutrient Agar (NA) plates, and the plates were incubated at 28°C for 24hr for colony emergence.

Figure 1: Field symptoms of rotten carrot.
Pathogenicity
The pathogenicity was proved by two methods, i.e., the wholecarrot- root method and carrot-slice-method as described previously [15]. In the whole-carrot-root method, the surface disinfected healthy carrots were injected with 250-300 μl of bacterial suspension (1×108 CFU mL-1) aseptically with the help of a sterilized syringe at its crown portion, then it was placed in a plastic bag along with wet cotton, and was incubated for 3-4 days at room temperature in a plant growth chamber. Whereas in the carrot-slice-method, the carrot root was cut into slices of 5mm thickness and was placed in a Petri plate and inoculated with 150-200 μl of bacterial suspension (1×108 CFUmL-1) at the cut surface (by pouring), then the Petri plates were placed in a plastic bag along with wet cotton and incubated for 3-4 days at room temperature in a plant growth chamber.
DNA isolation and primer synthesis
One of the isolates, AB1, was selected for taxonomic identification using the universal 16S rDNA gene. Bacterial cells harvested from the 24hr grown broth were used for bacterial genomic DNA isolation. The isolation of DNA was carried using the CTAB (Cetyltrimethylammonium bromide) as described previously [27]. The DNA was quantified spectrophotometrically and the Universal primer pairs for 16s rRNA gene, i.e., Fd1 (5'-AGAGTTTGATCCTGGCTCAG-3') and Rp1 3'-ACGGCTACCTTGTTACGACTT-5') [16] were synthesized at a commercial facility (Eurofins, Bengaluru, India).
PCR amplification
The PCR assay was conducted in a 20μl reaction mixture containing 10μl of 2X master mix (TaKaRa, Japan), 6μl of nucleasefree water, 10pmol/μl of forward primer, 10pmol/μl of reverse primer, and 50ng of DNA and performed in a thermal cycler (Eppendorfvapo. Protect, Germany). The PCR conditions included 94°C for 1min of denaturation, annealing of 60°C for 1min, and extension with 72°C for 1min 30s with 30 cycles of repetition. The amplified PCR product of 5μL was gel electrophoresed on 1% agarose, and the purified product was outsourced for sequencing (Eurofins, India). A BLAST search on the NCBI Gene Bank database [28] was used to identify the taxonomic identity. The nucleotide sequences of other strains of A. baumannii were retrieved from the NCBI GenBank and assembled using BioEdit Sequence Alignment Editor (Version 7.2.5) (Hall 1999). The evolutionary history was inferred using the Maximum Likelihood method and Tamura-Nei model (Tamura and Nei 1993) in the MEGA X software package (Version 10.1.7) (Kumar et al. 2018).
Biochemical characterization
The biochemical tests viz., KOH (3%), gram staining, growth at high temperature (37°C), muciodness, pigmentation on YDCA, growth on NaCl (5%) medium, catalase, oxidase, nitrate reductase, oxidative fermentation, acetoin, phenyl deaminase, urease production, amylase production was carried out. Utilization of carbohydrates like citrate, adonitol, delucitol, cellobiose, fructose, lactose, glucose, mannitol, maltose, sorbitol, sucrose, xylose was done according to Bergey’s Manual of Systematics of Archaea and Bacteria [29] and Laboratory guide for identification of plant pathogenic bacteria [30].
Results
Collection and isolation of bacterial isolates
A field survey was conducted in the carrot growing fields of Chintamani, Karnataka, India, during August 2020 indicated the incidence of soft rot disease in the surveyed field. Several carrot plants with wilting/collapse symptoms were observed in the field with a disease incidence of 10-12%. When the taproots of the symptomatic plants were observed after uprooting, they showed brown water-soaked lesions with rotting, and a prominent foul smell was also evident in all the symptomatic roots (Figure 1). The associated bacterium was isolated using a host (bell pepper)- enrichment technique. The bell pepper developed a water-soaked lesion around the pricking region when it was pricked after stabbing the diseased root whereas; no symptoms were produced when bell pepper was pricked after stabbing a healthy carrot root (Figure 2). After a standard bacterial isolation technique on NA media, bacterial colonies with circular, whitish, smooth, mucoid, round, convex, and medium-sized appeared after 48hr of incubation (Figure 3). Pure culture for four isolates was recovered, and one of the isolates, AB1, was selected for further analysis.
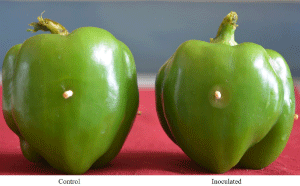
Figure 2: Enrichment host technique using bell pepper.
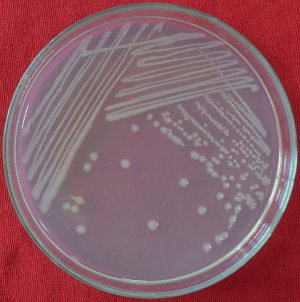
Figure 3: Colony morphology of A. baumannii isolate AB1.
Molecular identification and phylogeny
Using universal primers pairs, the PCR amplification of the 16S rDNA region of AB1 isolate yielded approximately an amplicon of 1400bp length. The nucleotide sequences of AB1 isolate obtained after sanger sequencing of the PCR amplified products were manually purified for trimming the low-quality reads. When the consensus sequences were subjected to the BLAST analysis, it showed 99.63 per cent identity with A. baumannii strain HAU423 (OK483367), thus confirming the taxonomic identity of AB1 as A. baumannii. The nucleotide sequences of the AB1 strain were deposited in the NCBI GenBank with an accession number OL872258. Further, a Maximum Likelihood phylogenetic tree constructed using 16S rDNA sequences indicated the close clustering of AB1 strain with other strains of A. baumannii isolated from soil, plant, human, and mosquito, indicating common ancestral origin irrespective of the host/source (Figure 4).
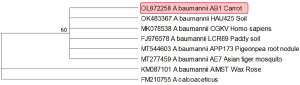
Figure 4: Phylogenetic analysis of A. baumannii isolate AB1.
Pathogenicity
This assay was conducted using both whole-root and sliced-root methods. In the whole-root method, the A. baumannii strain AB1 produced water-soaked lesions on carrot after 24hr of incubation. Later the lesion extended, and maceration of tissue was observed after 48hr, and complete rotting was observed at 72hr of incubation (Figure 5). In the sliced-root method, reddish to brownish watersoaked lesion was started at the inoculated point after 24hr, later the lesion gradually extended, leading to complete rotting (Figure 6). The re-isolation was done from both the methods, and recovered colonies showed the same characters as the original strain AB1.

Figure 5: Pathogenicity assay of A. baumannii isolate AB1 using whole-root
method.
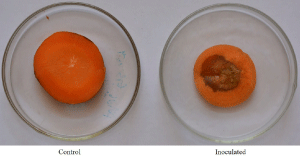
Figure 6: Pathogenicity assay of A. baumannii isolate AB1 using slice
method.
Physiological and biochemical characterization
Cells of A. baumannii strain AB1 showed round to rod-shaped morphology under microscope. It was non-motile, produced nonpigmented mucoid colonies on Luria-Bertani agar medium, and had no pigmentation on YDCA media. It showed a gram-negative reaction and formed thick viscous threads with 3% KOH. The bacterium was salt-tolerant (5% NaCl), and growth was observed at higher temperatures (37°C). The active culture showed negative oxidase activity, produced catalase and nitrate reductase, and possessed both oxidative and fermentative metabolism. A. baumannii was negative for acetoin production and phenylalanine utilization and positive for urease production. The bacterium used different carbohydrate sources like malonate, cellobiose, citrate, glucose, sorbitol, lactose, fructose, sucrose, xylose, and adonitol and failed to utilize mannitol, delucitol, and maltose. The results of biochemical and physiological characters are summarized in Table 1.
Biochemical test
A. baumannii (strain AB1)
Gram reaction
-
KOH (3%)
+
Growth at 37°C
+
5% NaCl
+
Colonies on YDCA
whitish
Muciodness
+
Motility
Non-motile
Oxidase
-
Catalase
+
Nitrate reductase
+
Oxidative fermentation
+/+
Acetoin
-
Phenylalanine deaminase
-
Urease
+
Malonate
+
Cellobiose
+
Citrate
+
Glucose
+
Sorbitol
+
Mannitol
-
Lactose
+
Fructose
+
Sucrose
+
Dulcitol
-
Maltose
-
Xylose
+
Adonitol
+
Table 1: Biochemical characteristics of A. baumannii.
Discussion
Several bacteria such as P. agglomerans, B. cepacia, E. cloacae, P. aeruginosa, and A. baumannii have been reported to infect diverse hosts belonging to the different kingdoms [31-35]. This crosskingdom infectivity is responsible for several emerging diseases in animals and plants [36]. Among them, A. baumannii has been reported from soil, plant, mosquito, human skin, upper respiratory tract, and gastrointestinal tracts, etc., and has been reported as an emerging pathogenic threat to public health [37]. Concerning plant association, A. baumannii has been associated with many vegetables and mainly disseminated by wild bird’s dropping [23]. It is found to show growth promotion activity in Pearl millet. Few Acenitobactor species like A. antiviralis and A. lactucae were found as endophytes associated with tobacco roots and lettuce plants, respectively [38,39]. However, it has also been reported as a plant pathogen associated with the top rot phase of the red stripe of sugarcane [24], dieback of mango [25], and tomato [26]. Therefore, it is essential to study the distribution of A. baumannii in the vegetables, especially those grown (carrot, radish, beetroot, leafy vegetables, etc.) in the urban ecosystem where human waste is frequently added to the soil.
Our field survey has identified the incidence of soft rot disease of carrot in the field. Our further pathogen isolation experiments indicated negative for the previously reported soft-rot pathogens (E. caratovora, P. carotovorum, and K. variicola, P. viridiflava, and P. marginalis) in the symptomatic and as well in the healthy samples. However, we could isolate A. baumannii bacterium from the diseased samples and be absent in the healthy samples indicating its role in the soft rot disease and symptoms. During isolation, to rule out the saprophytic microbiome in the symptomatic samples, we followed a host-enrichment technique using a healthy bell pepper fruit to purify/ isolate the pathogen from the diseased samples. One of the isolates, AB1, was further studied for its colony morphology, physiological and biochemical characterization, which were found to be similar to those reported previously for A. baumanniii [24], provided preliminary hints for the taxonomy of AB1 as A. baumanniii. Further, the taxonomy was confirmed through NCBI BLAST and phylogenetic analysis of the 16S rDNA gene sequences.
Taxonomically identified AB1 strain of A. baumanniii was tested for its pathogenicity on carrot using two independent methods. In both the methods of infectivity assays, A. baumannii produced watersoaked lesions leading to maceration and rotting of carrots, whereas the water inoculated carrot remains healthy, thus confirming A. baumanniii as the causal organism of carrot soft rot disease. Even though several previous reports have shown the association of A. baumanniii with plant diseases, we have provided conclusive evidence for its plant pathogenic potentials in this study. Previously, several bacterial pathogens such as E. caratovora [15], P. caratovorum [9], K. variicola [16], P. viridiflava, and P. marginalis [40] has been reported to cause the soft rot of carrot, and this study added one more bacterial pathogen, i.e., A. baumannii as a causal agent of soft rot disease of carrot.
A. baumannii is among the most troublesome pathogens globally and represents ESKAPE pathogens (ESKAPE: E. faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter spp.) [41]. In recent years, it has been designated as a “red alert” human pathogen, generating alarm among the medical fraternity, arising largely from its extensive antibiotic resistance spectrum. As vegetables are widely grown in all seasons and many regions, urban waste is frequently used in vegetable farming. There is an increased risk of movement of the cross-kingdom bacteria from human waste to cultivated fields and then to the consumers [42]. The evidence provided in this study indicated the vegetable-borne inoculum of A. baumannii can enter the food chain and, therefore, a potential threat to the public health.
Conclusion
Our study has established the pathogenic nature of a new pathogen, A. baumannii, in causing the soft rot disease of carrots in India. As the A. baumannii is an established cross-kingdom infecting pathogen, the vegetables act as a potential reservoir for this pathogen, and therefore, further research is required to manage this pathogen in the field and as well in the storage.
Declaration
Data availability: The authors confirm that the data supporting the findings of this study are available within the article
Author contributions: Conceived and designed the experiments: MKP. Performed the experiments: BSC. Contributed reagents/ materials/analysis tools: MKP. Wrote the manuscript: BSC, MKP, PD, PBP, SNB and SP. Edited the manuscript: PD, PBP, SNB, PME and MKP. All authors read and approved the manuscript for publication. The authors declare that they have no conflict of interest in the publication.
References
- Kirzinger MWB, Nadarasah G, Stavrindies J. Insights into Cross-Kingdom Plant Pathogenic Bacteria. Genes. 2011; 2: 980-997.
- Maki DG, Rhame FS, Mackel DC, Bennett JV. Nationwide epidemic of septicemia caused by contaminated intravenous products. Epidemiologic and clinical features. Am. J. Med. 1976; 60: 471-485.
- Govan JR, Hughes JE, Vandamme P. Burkholderia cepacia: Medical, taxonomic and ecological issues. J. Med. Microbiol. 1996; 45: 395-407.
- Min H, Li L, Yi-Xin W, Ho H, Peng-Fei H, Guo-Zhi L, et al. Pathogenicity of Klebsiella pneumonia (KpC4) infecting maize and mice. J. Integr. Agric. 2016; 15: 1510-1520.
- Nishijima KA, Wall MM, Siderhurst MS. Demonstrating pathogenicity of Enterobacter cloacae on macadamia and identifying associated volatiles of gray kernel of macadamia in Hawaii. Plant Dis. 2007; 91: 1221-1228.
- Masyahit M, Sijam K, Awang Y, Ghazali M. First report on bacterial soft rot disease on dragon fruit (Hylocereus spp.) caused by Enterobacter cloacae in peninsular Malaysia. Int. J. Agric. Biol. 2009; 11: 659-666.
- Takahashi Y, Takahashi K, Watanabe K, Kawano T. Bacterial black spot caused by Burkholderia andropogonis on Odontoglossum and intergeneric hybrid orchids. J. Gen. Plant. Pathol. 2004; 70: 284-287.
- de Baere T, Verhelst R, Labit C, Verschraegen G, Wauters G, Claeys G, et al. Bacteremic infection with Pantoea ananatis. J. Clin. Microbiol. 2004; 42: 4393-4395.
- Maisuria VB, Nerukar AS. Characterization and differentiation of soft rot causing Pectobacterium carotovorum of Indian origin. Eur. J. Plant. Pathol. 2013; 136: 87-102.
- De Haan EG, Dekker-Nooren TCEM, Bovenkamp GWVD, Speksnijder AGCL, Van Der Zouwen PS, Van Der Wolf JM. Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. Eur. J. Plant. Pathol. 2008; 122: 561-569.
- Lee DH, Lim JA, Lee J, Roh E, Jung K, Choi M, et al. Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum Pcc21 in Chinese cabbage. Microbiology. 2013; 159: 1487- 1496.
- Lee DH, Kim JB, Lim JA, Han SW, Heu S. Genetic Diversity of Pectobacterium carotovorum subsp. brasiliensis Isolated in Korea. Plant Pathol. J. 2014; 30: 117-124.
- Meng X, Chai A, Shi YX, Xie X, Ma Z, Li B. Emergence of bacterial soft rot in cucumber caused by Pectobacterium carotovorum subsp. Brasiliense in China. Plant Dis. 2017; 101: 279-289.
- Czajkowskiab R, Perombelond MCM, Van Veen JA, Van Der Wolf JM. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 2011; 60: 999-1013.
- Michalik B, Simon PW, Gabelman WH. Assessing Susceptibility of Carrot Roots to Bacterial Soft Rot. Hort Science. 1992; 27: 1020-1022.
- Chandrashekar BS, Prasannakumar MK, Puneeth ME, Teli K, Priyanka K, Mahesh HB, et al. First report of bacterial soft rot of carrot caused by KlebsiellaI variicola in India. NDR. 2018; 37: 21.
- Maragakis LL, Perl TM. Acinetobacter baumannii: Epidemiology, Antimicrobial Resistance and Treatment Options. Clinical Infectious Diseases. 2008; 46: 1254-1263.
- Carvalheira A, Ferreira V, Silva J, Teixeira P. Enrichment of Acinetobacter spp. from Food Samples. Food Microbiol. 2016; 55: 123-127.
- Monowar T, Rahman MS, Bhore SJ, Raju G, Satasivam KV. Secondary Metabolites Profiling of Acinetobacter baumannii Associated with Chili (Capsicum annuum L.) Leaves and Concentration Dependent Antioxidant and Prooxidant Properties. Biomed Res Int. 2019; 2019: 1-13.
- Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Asia. Clin. Microbiol. Rev. 2016; 30: 1-22.
- Ma C, McClean S. Mapping Global Prevalence of Acinetobacter baumannii and Recent Vaccine Development to Tackle It. Vaccines. 2021; 9: 1-25.
- Rokhbakhsh-Zanin, Farokh, Sachdev D, Kazemi-Pour N, Engineer A, Pardesi KR, et al. Characterization of Plant-Growth-Promoting Traits of Acinetobacter Species Isolated from Rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol. 2011; 21: 556-566.
- Dahiru M, Enabulele OI. Acinetobacter baumannii in Birds’ Feces: A Public Health Threat to Vegetables and Irrigation Farmers. Advances in Microbiology. 2015; 5: 693-698.
- Patro TSSK, Rao GVN, Gopalakrishnan J. Association of Acinetobacter baumannii with a Top Rot Phase of Sugarcane Redstripe Disease in India. Indian Phytopath. 2009; 59: 501-502.
- Khan IA, Khan A, Asif H, Jiskani MM, Muhlbach HP, Azim MK. Isolation and 16S rDNA Sequence Analysis of Bacteria from Dieback Affected Mango Orchards in Southern Pakistan. Pak J Bot. 2014; 46: 1431-1435.
- Kay E, Bertolla F, Vogel TM, Simonet P. Opportunistic Colonization of Ralstonia solanacearum-Infected Plants by Acinetobator sp. and its Natural Competance Development. Microb Ecol. 2002; 43: 291-297.
- Kumar MS, Sandhu AK, Genomic DNA Isolation from Fungi, Algae, Plant, Bacteria and Human Blood using CTAB. Int. J. Sci. Res. 2014; 3: 617-618.
- Basic Local Alignment Search Tool.
- Brenner DJ, Krieg NR, Staley JT, Garrity GM, eds. Bergey’s Manual of Systematic Bacteriology. Volume Two: The Proteobacteria. New York, USA: Springer. 2004.
- Schaad NW, Jeff J, Chun, W. Laboratory Guide for Identification of Plant Pathogenic Bacteria. St Paul, USA. 2001.
- Cruz AT, Cazacu AC, Allen CH. Pantoea agglomerans, a Plant Pathogen Causing Human Disease. J. Clin. Microbiol. 2007; 45: 1989-1992.
- Bernier SP, Silo-Suh L, Woods DE, Ohman DE, Soko PA. Comparative Analysis of Plant and Animal Models for Characterization of Burkholderia cepacia Virulence. Infect. Immun. 2003; 71: 5306-5313.
- Barcia-Gonzalez T, Saenz-Hidalgo HK, Silva-Rojas HV, Morales-Nieto C, Vancheva T, Koebni R, et al. Enterobacter cloacae, an Emerging Plant- Pathogenic Bacterium Affecting Chili Pepper Seedlings. Plant Pathol. J. 2008; 34: 1-10.
- Walker TS, Bais HP, Deziel E, Schweizer HP, Rahme LG, Fall R, et al. Pseudomonas aeruginosa-Plant Root Interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004; 134: 320-331.
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008; 31: 538-582.
- Rahme LG, Ausubell FM, Cao H, Drenkard E, Goumnerov BC, Lau GW, et al. Plants and animals share functionally common bacterial virulence factors. PANS. 2000; 97: 8815-8821.
- Joshi SG, Litake GM. Acinetobacter baumannii: An emerging pathogenic threat to public health. World J. Clin. Infect. Dis. 2013; 3: 25-36.
- Lee JS, Lee KC, Kim KK, Hwang IC, Jang C, Kim NG, et al. Acinetobacter antiviralis sp. nov., from tobacco plant roots. J. Microbiol. Biotechnol. 2009; 19: 250-256.
- Rooney AP, Dunlap CA, Weiler LBF. Acinetobacter lactucae sp. nov., isolated from iceberg lettuce (Asteraceae: Lactuca sativa). Int. J. Syst. Evol. 2016; 66: 3566-3572.
- Godfrey SAC, Marshall JW. Identification of cold-tolerant Pseudomonas viridiflava and P. marginalis causing severe carrot postharvest bacterial soft rot during refrigerated export from New Zealand. Plant Pathol. 2002; 51: 155- 162.
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 2008; 197: 1079-1081.
- Falomir MP, Gozalbo D, Rico H. Coliform bacteria in fresh vegetables: from cultivated lands to consumers. Current Research, technology and Education Topics in Applied Microbiology and Microbial Biotechnology. 2010; 1175-1181.