
Case Report
J Bacteriol Mycol. 2022; 9(1): 1195.
Intranasal Rhinosporidosis: A Case Report of a Rare Disease
Kenna E¹*, Djomou F², Ngo Nyeki A², Vouffo F³, Weledji E¹, Njock R²
1Regional Hospital Limbe, S.W. Region, Cameroon
2Department of Ear, Nose & Throat, Faculty of Medicine, Yaounde, Cameroon
3University Teaching Hospital, Yaounde, Cameroon
*Corresponding author: Kenna Ernest, Regional Hospital Limbe, S.W. Region, Cameroon
Received: January 19, 2022; Accepted: February 12, 2022; Published: February 19, 2022
Abstract
Rhinosporidiosisis a rare mycotic disease caused by Rhinosporidiumseeberithat affects both humans and animals. We report the case of a 12-year-old girl who presented with a six month history of a progressive right nasal obstruction and ipsilateral epistaxis. Clinical examination revealed a pedicled polyp on the underside of the right lower cornea of the right nostril. Histological examination of the excised polyp confirmed a rhinosporidosis. Follow-up after 2 years showed no recurrence. Although rare, it is an important differential diagnosis of a nasal polyp for a general physician to be aware of.
Keywords: Nose; Rhinosporidiosis
Introduction
Rhinosporidiosis is a rare mycotic disease caused by Rhinosporidiumseeberi. It affects both humans and animals, especially pets [1]. Human infection occurs through contact with stagnant fresh water harbouring the fungi. Although it is widely considered as a fungus, Rhinosporidiumseeberi has never been cultured in the laboratory, but microscopy shows the morphological characteristics of fungi and protozoa [2]. Direct human-to-human contamination has not been demonstrated and the disease manifests itself as a polypoid lesion, most often intranasal in location. Rare cases have been described on the conjunctiva, nasopharynx, rectum, vagina, urethra, mouth and skin. Rhinosporidiosis is worldwide. It is endemic in India, Sri Lanka and South America which account for 85% of reported cases [1,3]. In Africa, until 1990, the majority of reported cases came from Uganda (54.8%) and South Africa (18.3%). This is the third reported case in Cameroon [4,5]. Because of the lack of published reports this may give a false impression of the disappearance of the disease in the country
Case Presentation
A 12-year-old female child was admitted electively for management of a progressive right nasal obstruction and homolateral epistaxis. Physical examination without preparation of the right nasal fossa showed a pedicled polyp attached to the underside of the right lower nasal turbinate. This excrescence, which had a rosaceous appearan bled easily on contact and was non-tender. The left nostril was free and the rest of the ear, nose and throat ENT, and general examination were normal. The clinical impression was a benign tumor of the right nasal fossa. After right nasal preparation with topical xylocaine naphazozoline, there was a spontaneous reduction in the volume of the mass, which facilitated the resection at its base using electrocautery. This coarsely lamellar mass was sent in a 10% formaldehyde solution for a histopathological analysis [6]. The histology report showed macroscopically a pinkish-colored friable tissue fragment of 2.3cm in length. The microscopic analysis showed a squamous or respiratory epithelium with focal hyperplasticity without cytological atypia of its surface. The chorion is the site of a dense lymphocytic granuloma, without epithelioid cells and within which numerous thick-walled sporangia of different ages are present. Some are intact and filled with endospores. The others have a parietal opening through which endospores are released into the surrounding tissue (Figure 1). The last, more numerous ones are empty and keep their walls thick (Figure 2).
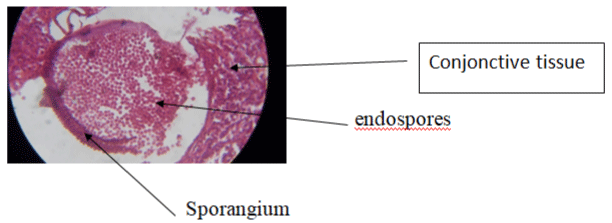
Figure 1: Sporangium releasing endospores in the surrounding tissue H&E x100.
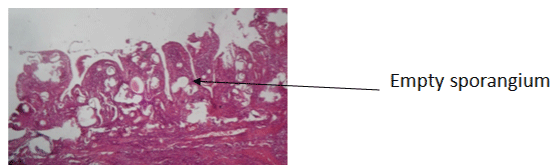
Figure 2: Empty sporangia in chronic lymphocytic dense inflammatory tissue H&E x40.
On the basis of these clinicopathological data, the diagnosis of intranasal rhinosporidiosis was made. The patient no longer received any additional treatment and a six-monthly follow-up plan was set up. No local recurrence was observed after two years.
Discussion
Rhinosporidiosis is a rare mycotic infection, affecting both humans and pets and caused by Rhinosporidiumseeberi. It has been known for about 100 years and the first cases were diagnosed in Argentina [7]. It was originally classified as a fungus due to the fact that it is coloured by fungal stains such as Gomoriand the PAS. Subsequently, it has been shown to have both morphological characteristics of fungi and protozoa [8,9]. More recently, and on the basis of phylogenetic analyses, the Mesomycetozoa class has been selected in taxonomy to contain this organism, and is now classified as a protozoan parasite [1,7]. This class has two orders: Dermocystida and Ichthyophonida. In the order Dermocystida, there are two families, including Rhinosporideaceae which includes Rhinosporidiumseeberi. The disease affects both men and women with a female/male ratio of 1:5 [10]. The reason for the high prevalence in male is not known. Some authors suggest it linked to hyperactivity in men in both field and aquatic work [11]. All age groups are concerned. Cases have been reported in children [10], adolescents, adults [3,12] and older people [7]. A Brazilian series [10], showed an age range of 7-24 years with an average age of 14 years. Although stagnant waters are the natural habitat of Rhinosporidiumseeberi, not all patients have a history of contact with such waters, as for example our patient who lives in an urban area and uses running water distributed by an approved company. The infection occurs through a breached cutaneous-mucosal barrier. The spread of the infection is by (i) Autoinoculation after rupture of the sporangia and release of endospores, (ii) Through the haematogenous route leading to metastases or even septicaemia, (iii) By lymphatic and (iv) Sexual route [13]. In view of this pathogenesis, it is understandable that there may be multiple locations, thus determining the multiplicity of clinical manifestations. The main site of the disease is the nose in 70-92% [3,10] of cases, followed in order of frequency by the conjunctival mucosa [14,15]. Other locations have also been reported such as the outer ear, parotid gland, bones, cavum, larynx [5], trachea [16], genitals, rectum [7] and skin. Skin location can be primary [12,17] or secondary. Regardless of the site of the disease, in the absence of concomitant disease, the biologic assessment is normal. Nasal rhinosporidiosis is manifested by occasional epistaxis, rhinorrhea, nasal pruritus, sneezing and a blocked nose. These symptoms can last from a few months to several years, sometimes for up to five years [7] and affect the left nostril in the majority of cases [10], although the reasons for this are not known. In contrast to inflammatory polyps which originate in the middle meatus, nasal rhinosporidiosis most often involves the anterior nostrils, the lower concha, the septum or the floor. Histopathologically, rhinosporidiosis can be confused with coccidioidomycosis (caused by Coccidioidesimitis) which is histologically characterized [19] by a granulomatous epitheliogigantocellular reaction sometimes centred on caseous necrosis. Its sporangia contain a variable number of endospores, but rarely up to twenty. The effective medical treatment alone against rhinosporridiosis remains controversial. The most commonly used therapeutic strategy [1,2,7] consists of surgical resection of the polyp’s implantation base followed by cauterization of the surrounding tissue to destroy any residual sporangia and endospores that may be present. This significantly reduces the local recurrence rate. In addition to this treatment, some authors believe that antifungal drugs such as Dapsone [7], ketoconazole [3] and others should be combined for several months. Hence the need for a comparative study of these two strategies. Our patient benefited from cauterization of the base of the lesion rather for hemostatic purposes, as the diagnosis of rhinosporidiosis was not mentioned in the preoperative phase. No drug treatment was given once the diagnosis was confirmed and there was no local recurrence after 2.5 years.
Conclusion
Rhinosporidiosis is an important differential diagnosis of a nasal polyp. It should be taken into consideration as it would influence medical or surgical treatment.
References
- Marc G, Eric C, Martin D, Jean M, Bernard D, Bernard R, et al. Médecinetropicale. 5eédition, Paris: Flammarion. Troisième partie : Maladies mycosiques, chapitre 2 : mycoses profondes. 1972; 286.
- Ngamdu YB, Ngadda HA, Kodfya AM, Sandabe MB, Isa A, Garandawa HI. Rhinosporidiose nasale: Un rapport de cas et une revue de la littérature. JCR. 2014; 4: 26-28.
- Ondzotto G. Rhinosporidioseendonasale: Présentation du premier cas observé au Congo. Bull SOC Pathol Exot. 2002; 95: 78-80.
- Ravisse P, Le Gonidec G, Moliva B. Présentation des deux premiers cas de rhinosporidiose observés au Cameroun. Bull SOC Pathol Exot. 1976; 69: 222-224.
- Belat S. La rhinosporidiose (à propos d’un cas de localisation laryngée découvert au Cameroun). Thèse, Bordeaux. 1981: 353.
- Ordre professionnel des technologistes médicaux du Québec. Guide d’anatomopathologie d’Août. 2014.
- Shukla D, Bineeta K, Madhumita B, Neelima G, Rumpa S, Lakshmi V, et al. Rhinosporidiose chez l’homme: nouvelles interprétations et revue de la littérature de cette maladie énigmatique. Médical Mycology. 2011; 49: 311- 315.
- Err H, Ajello L, Taylor JW, Arseculeratne SN, Mendoza L. Analyse phylogénétique des 18 s de Rhinosporidiumseeberi petite sous-unité d’ADN ribosomique groupes cet agent pathogène parmi les membres de la protoctistanMesomycetozoa clade. J clin microbiol. 1999; 37: 2750-2754.
- UN Hluwalia KB. Nouvelles interprétations de la rhinosporidiose, maladie énigmatique des neuf dernières décennies. J submicrosccytolpathol. 1992; 24: 109-114.
- Francilio AA, Laisson De MF, Jaqueline DP, George Castro FDM, Joyce SL, Fabio FS, et al. Rhinosporidiose: La plus grande série de cas au Brésil. Rev Soc Bras Med Trop. 2016; 49: 473-476.
- Kaluarachchi K, Sumathipala S, Eriyagama N, Atapattu D, Arseculeratne S. Identification de l’habitat naturel de Rhinosporidiumseeberi par hybridation in situ. J Infect Dis Antimicrob Agents. 2008; 25: 25-32.
- Deshpande AH, Agarwal S, Kelkar AA. Rhinosporidiose cutanée primaire diagnostiquée par la cytologie du produit de cytoponction à l’aiguille fine: Rapport d’un cas avec revue de la littérature. Diagn Cytopathol. 2009; 37: 125-127.
- Capoor MR, Khanna G, Rajni, et al. Rhinosporidiose à Dehli au nord de l’Inde: Séries de cas d’une zone non endémique. Mycopathologia. 2008; 168: 89-94.
- KaimboWa KD, Parys-Van GR. Rhinosporidioseconjonctivale: Un rapport d’un cas Congolais. Bull. Soc. Belge Ophtalmol. 2008; 22: 309-310.
- Sood NN, Raq SN. Granulome de rhinosporidiose de la conjonctive. Br. J Ophtalmol. 1967; 51: 61-64.
- Rajeev P, Sandeep B, Mandal AK, Berry N. Rhinosporidiose trachéale isolée : rapport d’un cas. Indian J. Otolaryngol. Head Neck Surg. 2000; 52: 380-381.
- Madhavan M, Ratnalar C, Mehdiratta KS. Rhinosporidiose du front (rapport d’un cas). J postgrad Med. 1978; 24: 235-236.
- Anabible-Dr Michels-coccidioïdomycose. 2009. Coccidioïdomycose.