
Research Article
J Bacteriol Mycol. 2022; 9(2): 1198.
Endophytic Yeasts in Apple Fruits of Cultivated and Wild Growth Forms: Total Diversity and Occurrence of Opportunistic Species
Kachalkin AV1,2, Glushakova AM1,3* and Venzhik AS1
1Department of Biology and Geology, M.V Lomonosov Moscow State University, Moscow, 119234
2Department of Soil Science, G.K Skryabin Institute of Biochemistry and Physiology of Microorganisms of RAS, Pushchino, 142290
3I.I Mechnikov Research Institute of Vaccines and Sera, Moscow, 105064
*Corresponding author: Anna M. Glushakova, M.V. Lomonosov Moscow State University, Moscow, 119234, Russia
Received: July 05, 2022; Accepted: August 04, 2022; Published: August 11, 2022
Abstract
Background: Apples are widely distributed in different geographical zones and are an important part of human diet. They are a popular subject for research and development of new technologies for biological protection of fruits against phytopathogens. Among yeasts, true phytopathogens are practically unknown. But among them there is a group of clinically significant species of the genus Candida. The development of these species in fruits can affect human health. Primarily, this concerns people with a weakened immune system and a genetic predisposition to mycogenic allergies. The presence of opportunistic yeasts is particularly likely in natural substrates under anthropogenic impact. This study is devoted to the comparison of endophytic yeasts of apples grown industrially and growing wild in urban areas.
Results: Endophytic yeasts were detected in 80% of samples. The average yeast abundance depended (F=24.26; p < 0.01) on the sugar concentration in tissues. At a content of 7-12 °Bx, the average yeast abundance was 4.96±1.07 × 10³ CFU/g; at 12-18 °Bx, 9.61±1.09 × 10³ CFU/g. A total of 33 yeast species were isolated from apples. The greatest number of endophytes was observed in wild apples. Detection the opportunistic yeast Candida parapsilosis distinguished yeast complexes of wild apples from commercial industrial products. The relative abundance of C. parapsilosis in apples collected in the urban area exceeded 30%.
Conclusion: The data on the high abundance of C. parapsilosis in endophytic yeast communities of apples collected in urban areas allow us to make a preliminary suggestion to avoid the consumption of such fruits.
Keywords: Malus domestica; Apples; Fruit; Endophytic yeasts; Candida parapsilosis
Introduction
The apple tree (Malus Mill.) is widely distributed in a variety of geographical zones and is one of the most important, popular, attractive and widely consumed fruit plants. Currently, there are more than 15,000 apple tree cultivars worldwide, and breeding of new cultivars continues unabated [1–5]. Cultivars of Malus domestica have been successfully used for commercial cultivation, home gardens, and landscaping in urban areas.
Apple fruit is of particular interest as a subject of study related to the development of new technologies for fruit storage and transportation [6]. Endophytic yeast strains with pronounced fungicidal activity (Aureobasidium pullulans, Debaryomyceshansenii, Metschnikowiapuicherrima, M. sinensis, Wickerhamonecesanomalus) against the most common phytopathogens of fruits (Aspergillus niger, Botrytis cinerea, Colletotrichum capsica, Moniliniafructicola, Rhizopus nigricans and others) have been repeatedly found in the pericarp of apples [7–11]. The internal tissue of apple fruits is also an interesting substrate for the isolation and description of new yeast species [12].
The most common yeast species observed both on the surface and inside apples and some other juicy fruits are Aureobasidium pullulans, Buckleyzyma aurantiaca, Curvibasidium cygneicollum, Cysto filobasidium infirmo miniatum, Debaryomyces hansenii, Galactomyces candidus, Hanseniaspora guilliermondii, H. uvarum, Metschnikowia pulcherrima, Naganishia albida, Pichia kluyveri, P. kudriavzevii, Papiliotrema laurentii, Rhodotorula minuta, Rh. glutinis, Saccharomyces cerevisiae, and Sporobolomyces roseus [13–14].
However, in urban ecosystems exposed to complex anthropogenic influence, changes in endophytic yeast communities may occur [15]. The opportunistic yeast species Candida parapsilosis was found in the internal tissues of Malus domestica and Pyrus communis fruits grown in Moscow throughout the formation and ripening period, reaching a maximum relative abundance in ripe fruits. The presence of opportunistic Candida yeasts is an indicator of environmental conditions and anthropogenic pressure [16].
In this study, we compared the abundance and taxonomic structure of endophytic yeast complexes associated with industrially grown apple fruits in different countries and those growing wild in private gardens in urban environments.
Materials and Methods
Study Location and Dampling
Apples for the study of the abundance and endophytic yeasts’ taxonomic structure were purchased from trade networks in the Moscow region (imports from Argentina, Belarus, China, Chile, Serbia, Turkey, and supplies from Russia), collected on the territory of the private gardens near Moscow city, and in the urban area along roadsides and in city parks in Moscow. The study was conducted in 2019. A total of 553 apple fruits were analyzed.
Microbiological Analyses and Species Identification
The abundance and taxonomic structure of yeasts were studied using a surface plating method on solid media.
To study endophytic yeast communities, fruits were treated according to the following scheme: 70% ethanol, 30 min; 2% sodium hypochlorite, 30 min; 70% ethanol, 30 s; and washing in sterile distilled water for 10 min [17,18]. After the exocarp was removed with a sterile scalpel, the internal tissues were cut out, homogenized, and poured with sterile water to obtain a 1:10 dilution. The suspensions were vortexed on a Multi Reax Vortexer (Heidolph Instruments, Germany) for 15 minutes at 2,000 rpm. Three suspensions were prepared for each apple. Thus, 1659 prepared suspensions were plated in two replicates each on glucose-peptone-yeast extract (GPY) agar (20 g/L glucose, 10 g/L peptone, 5 g/L yeast extract, 20 g/L agar) supplemented with chloramphenicol (500 mg/L) to prevent bacterial growth. A total of 3318 plates were incubated at 22°C for 5-7 days. The grown yeast colonies were classified into morphological types using a dissecting microscope, and the number of colonies of each type was counted. From each morphotype, 5 to 7 colonies were isolated into a pure culture.
Identification of yeast species was based on the ITS rDNA nucleotide sequence. DNA isolation and PCR were performed according to the previously described procedure [15]. DNA sequencing was performed using the Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, USA) with subsequent analysis of the reaction products on an Applied Biosystems 3130xl Genetic Analyzer at the facilities of Evrogen (Moscow). For sequencing, the ITS5 primer (5’- GGA AGT AAA AGT CGT AAC AAG G) was used. For species identification, nucleotide sequences were compared with those in public databases using the BLAST NCBI (www.ncbi.nlm.nih. gov) and the MycoID (www.mycobank.org) tools.
Analysis of Sugar Content
Total sugar content (measured by soluble solids concentration (SSC)) in fruit juice was estimated using a Milwaukee MA871 refractometer.
Data Analyses
The relative abundances of species were calculated as share (%) of colonies that appeared on the plates. Statistical data processing and graphical presentation of the obtained results were carried out using Excel 2010 (Microsoft, USA) and Statistica 8.0 (StatSoft, USA) programs. The Fisher’s test was used for comparing average data on the yeasts abundance after determining normality of their distribution by the Shapiro-Wilk test. The bars on figure represent the standard deviations (SDs).
Results
Yeast Abundance
Endophytic yeasts were detected in 80% of the samples tested. The average yeast abundance in the internal tissues of ripe apples depended (F criterion, 24.26; p < 0.01) on the sugar concentration in the internal tissues of apples. At a sugar content of 7-12 °Bx, the average yeast abundance was 4.96±1.07 × 10³ CFU/g; at 12-18 °Bx, it was 9.61±1.09 × 103 CFU/g (Figure 1). It should be noted that “peaks” in yeast abundance were also observed in apples with average sugar concentration (10 °Bx).
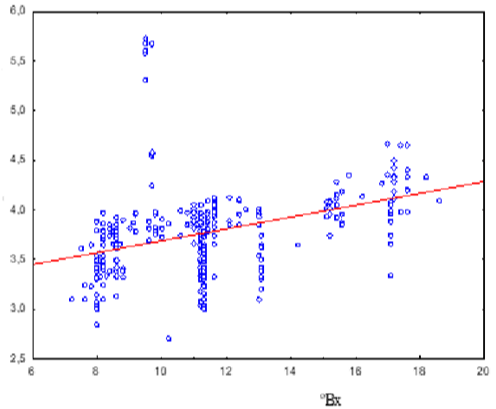
Figure 1: Average abundance of endophytic yeasts, lg(CFU/g) in apples with
different sugar content values, °Bx.
Detailed analysis of the abundance of endophytic yeasts in the internal tissues of applesas a function of sugar concentration (low and medium values - 7-12 °Bx and medium-high and high values - 12-18 °Bx) showed lower values ofaverage abundance of endophytic yeasts in commercially grown apples and their lower dependence on sugar concentration (Figure 2).
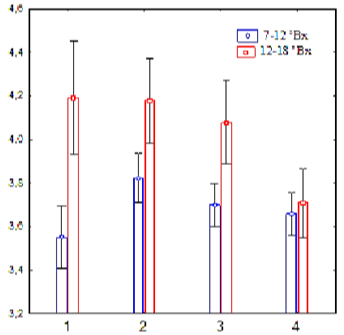
Figure 2: Abundance of endophytic yeasts, lg(CFU/g) in apples of different
growth forms and sugar content values (1 - along roadsides; 2 - city parks;
3 - private gardens; 4 - industrial products).
Diversity of Yeasts
A total of 693 yeast strains were isolated from all apples in this study. These isolates were identified to 33 species, 16 ascomycetes and 17 basidiomycetes. Half of the yeast genera were represented by only one species; yeasts of the genera Candida, Cystofilobasidium, Debaryomyces, Filobasidium, Kwoniella, Metschnikowia, Meyerozyma, and Vishniacozyma were represented by two species, and the genus Rhodotorula by three species. Only one yeast genus, Yarrowia, was represented by four species. The most common yeast species among apple endophytes were: Aureobasidium pullulans, Candida parapsilosis, C. zeylanoides, Cystofilobasidium capitatum, Debaryomycesfabryi, Filobasidium magnum, F. wieringae, Hanseniasporauvarum, Leucosporidiumscottii, Metschnikowia pulcherrima, Rhodotorula babjevae, Rh. mucilaginosa, and Yarrowia spp. (Table 1).
Yeast species
FQ, %
Ascomycota
Aureobasidiumpullulans
18.63
Candida parapsilosis
28.75
Candida zeylanoides
25.86
Debaryomycesfabryi
5.61
Debaryomyceshansenii
2.53
Dothiorasorbi
0.54
Hanseniasporauvarum
32.37
Metschnikowiachrysoperlae
0.54
Metschnikowiapulcherrima
47.20
Meyerozyma carribica
1.63
Meyerozyma guilliermondii
3.80
Pichia kluyveri
2.89
Yarrowiadeformans
9.95
Yarrowiadivulgata
4.34
Yarrowiagalli
14.10
Yarrowialipolytica
9.40
Basidiomycota
Bulleraalba
0.36
Cystobasidiumsp.KBP YE-0710
3.62
Cystofilobasidiumcapitatum
34.90
Cystofilobasidiummacerans
3.44
Filobasidiummagnum
5.97
Filobasidiumwieringae
9.40
Kwoniellaendophytica
0.18
Kwoniellapini
0.90
Leucosporidiumscottii
5.06
Moesziomycesaphidis
2.53
Papiliotrema horticolasp. nov.
0.18
Rhodosporidioboluscolostri
0.72
Rhodotorula babjevae
10.31
Rhodotorula glutinis
1.81
Rhodotorula mucilaginosa
8.50
Vishniacozymatephrensis
0.18
Vishniakozymacarnescens
1.27
Table 1: Frequency (FQ, %) of endophytic yeast species isolated from apples.
The highest species richness of endophytic yeasts was observed in wild apples collected in urban areas along highways and in city parks (20 species, predominantly Candida parapsilosis and Cystofilobasidium capitatum), and in private gardens near the city (17 species, predominantly Metschnikowia pulcherrima and Hanseniasporauvarum). Among industrial products, the highest species richness was observed in apples imported from Chile (12 species, predominantly Debaryomycesfabryi, Yarrowiagalli and Candida zeylanoides), and the lowest in apples imported from Serbia (2 species, predominantly Cystobasidiumsp.nov.). The average number of yeast species per apples examined was 11.4 (Table 2).
Wild product
Industrial product
Yeastspecies
City
Gardens
Argentina
Belarus
Chile
China
Russia
Serbia
Turkey
Aureobasidium pullulans
5.1
1.4
3.2
0.3
0.9
1.0
13.0
8.3
0.6
Bullera alba
0.1
–
–
–
–
–
–
–
–
Candida parapsilosis
39.3
–
–
–
–
–
–
–
–
C. zeylanoides
1.6
0.7
24.9
24.4
11.2
17.1
–
–
17.9
Cystobasidiumsp.nov
–
–
–
–
–
–
8.2
91.7
–
21.4
2.9
5.5
4.7
1.5
8.8
–
–
1.9
Cyst. macerans
0.2
1.9
–
–
–
–
–
–
–
Debaryomycesfabryi
–
–
5.8
12.5
45.6
14.0
–
–
11.1
D. hansenii
1.1
–
–
–
–
–
16.7
–
–
Dothiorasorbi
0.1
–
–
–
–
–
–
–
–
Filobasidium magnum
1,9
1.4
–
–
–
–
–
–
–
F. wieringae
5.6
4.6
–
–
–
Hanseniasporauvarum
4.7
13.5
3.1
6.7
4.2
2.6
–
–
0.7
Kwoniellaendophytica
0.4
–
–
–
–
–
–
–
–
Kw. pini
–
–
–
–
–
–
4.3
–
–
Leocosporidiumscottii
3.9
–
–
–
–
–
–
–
–
Metschnikowiachrysoperlae
–
–
–
–
–
–
6.6
–
–
M. pulcherrima
9.0
53.7
6.7
12.4
4.4
20.0
33.1
–
5.6
Meyerozyma carribica
–
–
1.6
–
1.6
11.5
–
–
–
Mey. guilliermondii
–
–
2.7
11.0
2.5
4.5
–
–
0.7
Moesziomycesaphidis
–
0.4
–
–
–
–
–
–
–
Papiliotrema horticolasp.nov
–
0.1
–
–
–
–
–
–
–
Pichia kluyveri
0.6
–
–
–
–
–
–
–
–
Rhodosporidioboluscolostri
–
–
–
–
–
–
16.6
–
–
Rhodotorula babjevae
0.2
0.2
14.2
14.1
7.6
4.4
–
–
8.0
Rh. glutinis
0.1
–
–
–
–
–
–
–
–
Rh. mucilaginosa
0.9
0.5
2.0
1.5
2.3
2.5
1.3
–
13.4
Vishniakozymatephrensis
–
–
–
–
–
–
0.1
–
–
V. carnescens
–
0.2
–
–
–
–
–
–
–
Yarrowia deformans
0.7
6.5
–
–
5.8
–
–
–
–
Y. divulgata
–
4.8
–
–
–
–
–
–
–
Y. galli
–
4.9
30.2
12.4
12.5
13.8
–
–
40.1
Y. lipolytica
3.1
2.4
–
–
–
–
–
–
–
Table 2: The average relative abundance (%) of endophytic yeast species isolated from apples of different growth forms.
Opportunistic Yeasts in Endophytic Yeast Complexes
Detection of strains of the clinically important (opportunistic) yeast Candida parapsilosis distinguished the endophytic yeast complexes of wild apples collected in the urban areas of Moscow from commercial industrial products imported from different countries (Table 2). The relative abundance of C. parapsilosis in apples collected in urban areas (along highways and in parks) was significant and exceeded 30%.
Isolation of New Yeast Species
In this study, strains of two new yeast species from the genera Cystobasidium (KBP YE -0710) (found in fruits imported from Serbia) and Papiliotrema (KBP YE -0283) (found in fruits collected from private gardens near the city) were observed. The first species is phylogenetically closely related to the species Cys. benthicum (93.9% similarity of D1/D2 rDNA domains) and is currently described. The second species is phylogenetically close to Papiliotrema aurea (ITS region rDNA similarity - 97.4%) and was recently described by us as Papiliotrema horticola [19].
Discussion
Total Abundance of Obtained Endophytes
While in fruits collected in urban ecosystems, the abundance of endophytic yeasts in apples showed an obvious dependence on the sugar concentration in the internal tissues, the average abundance of endophytic yeasts in industrial products was almost independent of the sugar concentration. Most likely, the development of yeasts in commercial products is limited by the use of fungicides in the agrotechnical treatment of apples. However, these techniques do not completely eliminate yeasts. Camatti-Sartori et al. (2005) have reported a similar pattern that chemical compounds, mainly fungicides, used in conventional and integrated production systems reduce populations of endophytic filamentous fungi and yeasts in apple trees.
Isolated Yeast Endophytes
Among the yeast species found in the internal tissues of apples, there are species that are typical epiphytes: Candida zeylanoides, Cystofilobasidium capitatum, Metschnikowia pulcherrina, Hanseniaspora uvarum, Rhodotorula babjevae, Rh. mucilaginosa, etc. But also species that are predominantly minor components in a variety of natural yeast complexes: Metschnikowiachrysoperlae. Moesziomyces aphidis, Vishniakozymacarnescens, Yarrowiagalli, etc. [20]. The highest diversity of endophytic yeasts was found in apples collected in urbanized areas compared to industrially grown fruits in different countries (Table 2). This study suggests that urbanization promotes invasion of yeast species and may increase species richness at the local level. A similar effect has been repeatedly observed in several other groups of organisms when comparing their species richness in natural habitats and in urbanized areas [21–22].Changes in community structure due to urbanization may affect various aspects of ecosystems. Extensive future research is needed to reveal the effects of urbanization on the diversity of endophytic yeast complexes. Of course, not all yeasts found in fruits can be classified as true or obligate endophytes that develop asymptomatically and only in internal tissues. We should consider the groups of yeasts that can live endophytically and consider the contaminated species separately. It is likely that yeasts with endophytic lifestyles include typical epiphytic and eurytopic yeasts for which transition to endophytic lifestyles in internal tissues is a strategy to avoid unfavorable environmental factors (solar radiation, desiccation, etc.). There is evidence of the ability of yeasts to invade internal tissues relatively quickly. It has been shown that after inoculation of Meyerozyma guilliermondii on the surface of tomato, yeast cells were found in the epidermal layer after 8 hours and in the parenchyma layer after 22 hours [23]. Another, more complicated situation arises when considering the contaminating species. Most likely, it is the opportunistic yeast Candida parapsilosis, which was found in the internal tissues of urban apples.
Candida Parapsilosis Observed in Urban Apples
Candida parapsilosis can be observed in domestic animals, insects, soil, marine environments, etc. [24–25]. In addition, C. parapsilosis is commensal yeast of humans that frequently colonizes the skin. However, it can become pathogenic when host defense mechanisms change [26–28]. Due to its ability to adapt to different host niches, it can cause systemic infections in immunocompromised individuals [29–30]. The high abundance of Candida parapsilosis may indicate unfavorable growing conditions for agricultural products associated with high anthropogenic stress [16]. For example, this species was found in contaminated soils in Moscow [31] and isolated from apples and pears growing in city parks [15]. Recently, it has been shown that environmental strains of Candida parapsilosis isolated from urban soils can be resistant to widely used antimycotics [32–34]. It cannot be excluded that strains from the internal tissues of apples growing in urban areas are also resistant. In addition, endophytic strains of opportunistic yeasts may possess virulence characteristics (e.g., produce hydrolytic enzymes) like clinical isolates [26]. In this case, consumption of apples collected in urban areas may not be safe, especially for immune compromised individuals who are genetically predisposed to the development of fungal diseases and mycogenic allergies.
New Yeast Species Found in Endophytic Yeast Communities
The internal tissue of apples is a promising substrate for the detection of new species of endophytic yeasts. In our previous study, we have already isolated and described a new species Kwoniellaendophytica A.M. Glushakova et Kachalkin [12] from the inner tissues. During this study, we found strains of two new yeast species, one of which we identified as Papiliotrema horticolasp.nov [19]. and the second, Cystobasidiumsp.nov. (KBP YE -0710) is currently being described. The collection of endophytic strains of species from apples is being studied to find strains that produce phytohormones and strains with fungicidal activity. After optimization, such strains can be used to produce biopreparations to stimulate growth and protect crops.
Conclusion
The data obtained on the high abundance of the opportunistic species Candida parapsilosis in endophytic yeast communities of apples collected in urban areas allow us to make a preliminary recommendation to avoid consumption of such fruits. This study provided a basis for further investigation of antibiotic resistance and phenotypic characteristics associated with virulence of endophytic strains of C. parapsilosis isolated from urban apples.
Author Statement
A.V. Kachalkin: Conceptualization; Data curation; Visualization; Writing original draft. A.M. Glushakova: Conceptualization; Data curation; Formal analysis; Methodology; Writing original draft. A.S. Venzhik: Data duration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cornille A, Giraud T, Smulders MJM, Roldán-Ruiz I, Gladieux P. The domestication and evolutionary ecology of apples. Trends in genetics: TIG. 2014; 30: 57-65.
- Cronin D, Kron P, Husband BC. The origins and evolutionary history of feral apples in southern Canada. Molecular Ecology. 2019; 29: 1776-1790.
- Gross BL, Wedger MJ, Martinez M, Volk GM, Hale C. Identification of unknown apple (Malus × domestica) cultivars demonstrates the impact of local breeding program on cultivar diversity. Genetic Resources and Crop Evolution. 2018; 65: 1317–1327.
- Janick J. The origins of fruits, fruit growing, and fruit breeding. Plant Breed Rev. 2005; 25: 255–321.
- Robinson JP, Harris SA, Juniper BE. Taxonomy of the genus Malus Mill. (Rosaceae) with emphasis on the cultivated apple, Malus domestica Borkh. Plant Systematics and Evolution. 2001; 226: 35–58.
- Doty SL. Endophytic yeasts: biology and applications. Symbiotic Endophytes: Springer. 2013: 335–343.
- Chand-Goyal T, Spotts RA. Enumeration of bacterial and yeast colonists of apple fruits and identification of epiphytic yeasts on pear fruits in the Pacific Northwest United States. Microbiological research. 1996; 151: 427-432.
- Gross S, Kunz L, Müller DC, Kron AS, Freimoser FM. Characterization of antagonistic yeasts for biocontrol applications on apples or in soil by quantitative analyses of synthetic yeast communities. Yeast (Chichester, England). 2018; 35: 559-566.
- Pawlikowska E, James SA, Breierova E, Antolak H, Kregiel D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek. 2019; 112: 1425-1445.
- Saravanakumar D, Ciavorella A, Spadaro D, Garibaldi A, Gullino L.Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol Technol. 2008; 49: 121–128.
- Zhang D, Spadaro D, Valente S, Garibaldi A, Gullino ML. Cloning, characterization and expression of an exoglucanasa gene from the antagonistic yeast, Pichia guilliermondii against grey mold on apples. Biological Control. 2011; 59: 284–293.
- Crous P, Luangsa-ard J, Wingfield M, Carnegie A, Hernández-Restrepo M, Lombard L, et al. Fungal Planet description sheets: 785–867. Persoonia : Molecular Phylogeny and Evolution of Fungi. 2018; 41: 238-417.
- Camatti-Sartori V, Silva-Ribeiro RTD, Valdebenito-Sanhueza RM, Pagnocca FC, Echeverrigaray S, Azevedo JL. Endophytic yeasts and filamentous fungi associated with southern Brazilian apple (Malus domestica) orchards subjected to conventional, integrated or organic cultivation. Journal of Basic Microbiology. 2005; 45: 397-402.
- VadkertiováR, Molnárová J, Vránová D,SlávikováE.Yeasts and yeast-like organisms associated withfruits and blossoms of different fruit trees. Can J Microbiol. 2012; 58: 1344–1352.
- Glushakova AM, Kachalkin AV. Endophytic yeasts in Malus domestica and Pyrus communis fruits under anthropogenic impact. Microbiology. 2016; 86: 128-135.
- Hagler AN. Yeasts as indicators of environmental quality. Biodiversity and Ecophysiology of Yeasts. The Yeast Handbook: Springer. 2006; 515–532.
- Isaeva OV, Glushakova AM, Garbuz SA, Kachalkin AV, Chernov IY. Endophytic yeast fungi in plant storage tissues. Biology Bulletin. 2010; 37: 26-34.
- Gai CS, Lacava PT, MaccheroniWJr, Glienke C, Araujo WL, Miller TA, et al. Diversity of endophytic yeasts from sweet orange and their localization by scanning electronmicroscopy. J Basic Microbiol. 2009; 49: 441–451.
- Crous PW, Cowan DA., Maggs-Kölling G, Yilmaz N, Thangavel R, Wingfield MJ, et al. Fungal planet description sheets: 1182–1283. Persoonia. 2021; 46: 313–528.
- Buzzini P. Yeasts in natural ecosystems. Diversity: Springer. 2017.
- Karasawa S. Comparison of isopod assemblages (Crustacea: Isopoda: Oniscidea) among four different habitats - Evergreen Forest, exotic bamboo plantation, grass and urban habitat. Pedobiologia. 2022; 91–92: 150805.
- McKinney ML. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 2008; 11: 161–176
- Infante E, Marquinez X, Moreno G. Tomato peel (Solanum lycopersicum L.) colonization by the endophyte yeast Candida guilliermondii (Castellani) Langeron et Guerra. Agronomía Colombiana. 2012; 30: 388–394.
- Silva S., Negri M., Henriques M., Oliveira R., Williams DW, Azeredo J.Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012; 36: 288–305.
- Trofa D, Gacser A and Nosanchuk JD.Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008; 21: 606–625.
- Abi-chacra A, Souza LO, Cruz LPS, Braga-Silva LA, Gonçalves DS, Sodré CL, et al. Phenotypical properties associated with virulence from clinical isolates belonging to the Candida parapsilosis complex. FEMS yeast research. 2013; 13: 831-848.
- Nosek J, Holesova Z, Kosa P, Gacser A, Tomaska L. Biology and genetics of the pathogenic yeast Candida parapsilosis. Current Genetics. 2009; 55: 497-509.
- Asbeck ECV, Clemons KV, Stevens DA. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Critical Reviews in Microbiology. 2009; 35: 283-309.
- Eggimann P, Garbino J, Pittet D.Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003; 3: 685–702.
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MJSM. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. Journal of medical microbiology. 2013; 62: 10-24.
- Tepeeva AN, Glushakova AM, Kachalkin AV. Yeast communities of the Moscow city soils. Microbiol. 2018; 87: 407–415.
- Akhapkina IG, Glushakova AM, Rodionova EN, Kachalkin AV. Colonization activity of Candida clinical isolates and their antibiotic sensitivity J Microbiol Epidemiol Immunobiol. 2020a; 97: 197–202.
- Akhapkina IG, Glushakova AM, Rodionova EN, Kachalkin AV. The effectiveness of antifungal agents against yeasts of Candida genus isolated in Moscow region. Antibiotics and chemotherapy. 2020b; 65: 16–22.
- Glushakova AM., Kachalkin AV,Akhapkina IG. The monitoring of sensitivity of natural strains and clinical isolates of yeast fungus to antimycotics. Klin Lab Diag. 2017; 62: 296–300.