
Rapid Communication
Austin Biochem. 2017; 2(1): 1007.
Both Hair Cortisol and Perceived Stress during Fall Exams Decrease after the Winter Break
Blodgett J¹, Walter D¹, MacKenzie JA², Gump BB³ and Bendinskas K¹*
¹Department of Chemistry, State University of New York College at Oswego, USA
2Department of Biological Sciences, State University of New York College at Oswego, USA
³Department of Public Health, Food Studies, and Nutrition, Syracuse University, USA
*Corresponding author: Bendinskas K, Department of Chemistry, State University of New York College at Oswego, 360 Shineman, 30 Centennial Drive, Oswego, NY, 13126, USA
Received: November 28, 2016; Accepted: February 21, 2017; Published: February 22, 2017
Abstract
Hair cortisol concentrations have been associated with a significant number of physical and psychological stressors; however, few longitudinal studies have been reported. In this pilot work, ten male and ten female college seniors were asked to report their perceived stress levels and had one centimeter of the posterior vortex hair collected during their college fall finals. The same procedure was repeated about a month later after the winter break. Hair cortisol was extracted with methanol and measured using ELISA. Our findings indicate that hair cortisol concentrations and perceived stress levels, while not associated with each other, decreased significantly after the winter break (both with p<0.0001). Hair cortisol concentrations before and after the break were associated (p<0.01).
Keywords: Perceived stress; Hair cortisol concentrations; Longitudinal; Young adults
Abbreviations
HCC: Hair Cortisol Concentrations; PS: Perceived Stress; BMI: Body Mass Index; ELISA: Enzyme-Linked Immunosorbent Assay
Introduction
Several recent reviews on Hair Cortisol Concentrations (HCC) in humans document the rapidly expanding and maturing field of study [1-6]. There is a consensus that HCC increases in subjects with certain health factors (e.g., Cushing’s syndrome, use of hydrocortisone, metabolic syndrome, diabetes, cardiovascular disease [7-10]) and under chronic/acute stressors (e.g., vigorous exercise, difficult employment situation, chronic pain) [11-14]. HCC associations with traumatic events and with perceived stress are complex and depend on a multitude of factors with several studies having reported contradicting results [15-20].
Several cross-sectional studies have measured HCC in younger adults. In 99 university students, HCC increase was significantly related to serious life events [16]. In 46 university students, vigorous physical activity increased HCC [21]. In a group of 24 students, HCC was lower in those who had one or two bereavement experiences relative to those with no bereavement experiences [22]. In a study with 42 students, elevated HCC was associated with lower depressive symptoms and lower perceived stress [23]. Few longitudinal cortisol studies are available to date. The first longitudinal study on cortisol was published in 1999 where blood cortisol levels were found to increase across the weeks of gestation in pregnant adolescents [24]. Also, salivary cortisol and HCC were assessed longitudinally in 21 pregnant women, and both measures rose during pregnancy as expected [25]. Saliva cortisol was measured before and after a written examination in 11 graduate students, and higher cortisol was associated with higher stress before examination and lower exam grades [26]. In 52 females, blood cortisol levels were assessed three times at 6-month intervals and cortisol was positively related to concurrent general and social anxiety [27]. Cortisol was measured upon enrollment, immediately after enrollment, and 3 and 6 months after enrollment in school in 59 children. Salivary cortisol was increased 3 months after enrollment, indicating that children reacted to the challenge of school later rather than initially [28]. HCC were measured when entering school and 2 months after in 42 children; HCC were higher at the later date, especially for fearful kids [29]. In 151 patients with structural heart disease, HCC was measured at baseline and at 12-week follow-up in a randomized controlled trial of mindfulness training. At the base levels, HCC was associated with BMI, respiratory rate, and the physical summary score. After 12-weeks, HCC change was associated with the mental summary score and diastolic blood pressure [30].
Materials and Methods
Our study was designed to provide strong evidence of longitudinal HCC changes in young adults. The State University of New York College at Oswego Institutional Review Board approved this work. Traditional validated HCC assessment protocols were used [31-35], and no additional validation experiments were performed for this brief communication. The procedures are described in detail below. Of importance for longitudinal HCC measurements, intra-individual HCC stability [36] must be noted.
Questionnaire
Ten female and ten male senior chemistry students participated in this study at two different times: once during the finals week of the fall semester, when students are expected to be very busy and stressed, and again during the first week of the spring semester, when students are expected to be relaxed after a month of resting. The participant sample was very homogenous (same age students equally split by gender). Each participant signed a consent form saying they knew the benefits and risks of this study before the research project began. At both sample collection times, the students were given a questionnaire in which they had rated their Perceived Stress (PS) level on a tenpoint scale, from “not at all” to “very much”.
Hair collection and storage
Approximately 50-100 mg hair of one cm in length from the posterior vortex was cut with surgical scissors from as close to the scalp as possible and weighed on an aluminium weighing boat. The sample was stored in an envelope at room temperature in a cabinet until extraction. The timing of measurements (about a month apart) and the fact that we studied one cm of hair closest to scalp were intentional and coincide with observations that hair in European Americans grows approximately one cm/month [1-3,6].
Hair extraction procedure
Approximately 50 mg of hair was weighed in a 15×45 mm Fisher brand vial with a rubber-lined cap. The hair was washed twice with one mL of isopropyl alcohol, vortexed for 2 minutes, and the solution was discarded. The hair was submerged into one mL of methanol, vortexed for 30 seconds, and placed into a 55°C water bath overnight. The samples were sonicated for 60 minutes. Methanol was transferred to a micro centrifuge tube and evaporated under a gentle nitrogen stream. Extracts were resuspended in 80 μL of assay diluent that came with the Salimetrics cortisol ELISA kit and vortexed for 30 seconds immediately before ELISA procedure was performed.
For the confirmation of results, hair from all participants was pooled for the two collection times (exams and after the break), finely cut and triple- extracted (with methanol- acetone- methanol) [32].
ELISA
ELISA protocol was provided by Salimetrics. Duplicate 25 μL samples were analyzed. The plate was read at 450 nm with a KC Junior Bio Tek plate reader. Concentrations of the controls and hair samples were determined by interpolation using 4-parameter nonlinear regression curve fit provided by KC Junior. If duplicate readings were different by more than 10%, 25 μL of the sample were reanalyzed. The intra-assay precision was reported by Salimetrics as under 7%, the inter-assay precision under 11%, spiked recovery 97- 109%, the minimal concentration of cortisol that can be distinguished from 0 was 0.007 μg/dL in the solution, and the only cross-reactivity know was with dexamethasone, an anti- inflammatory medication. Samples from an individual from the finals week and after the break were analyzed on the same ELISA plate to avoid the batch effect.
Results
With the exception of our intentional sampling of equal numbers of males and females, the study population was homogeneous: ages of all participants were very similar (seniors in a college with no non-traditional students participating), the race was 90% European American (only one African heritage female and one Asian heritage male participated, which was typical for a central New York college).
Changes in perceived stress and hair cortisol are shown in (Figure 1). Among 20 HCC and 20 PS values, one value in each set was identified as a statistical outlier in a G-test and was not included in calculations. Both, HCC and PS decreased almost two-fold from the time of finals to the time after the break.
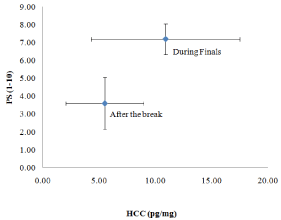
Figure 1: Longitudinal Changes in Hair Cortisol Concentrations (HCC)
and Perceived Stress (PS) in College Students. HCC and PS on average
decreased about two-fold after the finals (both with p<0.0001). Errors are
shown as one standard deviation.
Average HCC decreased from 10.9 pg/mg to 5.5 pg/mg. While there was a noticeable difference between individuals (yielding a high standard deviation for this measure), out of 19 participants, only one sample slightly increased in value while eighteen decreased. The change in HCC from finals to after break was found to be highly significant in a paired t-test (t (18) = 4.64, p < 0.0001).In an analysis of variance (ANOVA) with gender as the between-subject factor, change in HCC from exam to break did not differ as a function of gender, F (1,17) = 0.01, p>0.25. Log-transformed HCC value analyses showed identical patterns.
Reported perceived stress decreased in all subjects. The average PS decreased from 7.2 to 3.6 on a ten-point scale. As with HCC, this change from finals to after break was found to be highly significant in a paired t-test (t (18) = 10.83, p < 0.0001). It should be noted that even though HCC and PS both decreased, neither the HCC and PS ratio of values (exam/post break) nor the change of these values over time (exam-post break) was statistically correlated to each other (p> 0.25). In an ANOVA with gender as the between-subject factor, change in PS from exam to break did not differ as a function of gender, F (1,17) = 0.35, p>0.25.
Controlling for gender, HCC values during the final exams were significantly correlated with values after the winter break, r = 0.67, p = 0.004 (Figure 2); that is individuals who had higher HCC during the finals relative to other individuals tended to have HCC higher relative to the same individuals after the break as well.
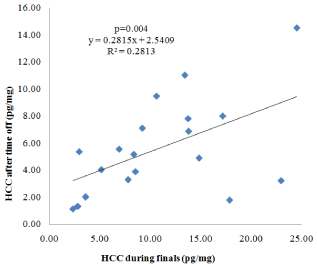
Figure 2: Intra-individual Stability of Hair Cortisol Concentrations (HCC).
Individuals with higher HCC during the finals tended to have higher HCC after
the time off too (p=0.004).
Pooled samples of hair that were finely-cut and triple-extracted showed exactly the same HCC decrease as described above (results not shown).
Discussion
The decrease of PS and salivary cortisol in 11 graduate students after a written exam was previously documented [26]. In that regard, our results are exactly as expected. The only difference was that we measured HCC, not salivary cortisol in our study. In addition, multiple studies indicate that humans similarly handle major physiological or psychological stress, and the organismal coping with that stress can be effectively measured by the increase in HCC [1-3,6]. Thus, our results indicate that what students experience during the month leading to finals is equivalent at their young age to a major stressor or a serious life event.
The fact that HCC and PS changes were not statistically associated was not surprising. Although both went down, the amount they went down or their ratios were not correlated. In other words, all students reacted to finals (they become stressed), and while some reacted more psychologically, some reacted more physiologically; however, these two measures do not necessarily go hand-in-hand since people who react physiologically are not necessarily the same ones who react psychologically[37]. Indeed, the literature is full of examples showing that PS and even traumatic events are not necessarily associated with HCC, and sometimes patterns found in one study contradict those found in another [1-3,6].
Statistical intra-individual stability of HCC has been well documented [36]. Genetic factors and long-term stressors affect HCC; and even though stress before and during finals and time off affects HCC significantly, other continuing influences likely define whether HCC are high or low in an individual at both times of testing [36].
The weaknesses of this rapid communication include the following. 1) the study included a relatively small number of participants (N=20) at two different periods of time; the statistical tests performed were based on the existing sample size, as such, the fact that significant differences were still found suggests a large effect size and therefore a finding of importance. 2) The researchers relied on the Salimetrics Cortisol ELISA kits and the standard HCC determination methods without additional validation experiments; nevertheless, data in (Figure 2) indicate that our results were consistent and as expected according to the literature. 3) In all longitudinal HCC literature, to our best knowledge, there is no evidence of the change or the lack of the change of cortisol concentration in properly stored hair; such experiments must be performed in the future in the field. Also, if there was a decrease in cortisol concentration over the time of storage since our results indicate that cortisol was about two-fold higher during finals as compared to after the break, then the actual effect might have been even larger than we documented.
Conclusion
The weeks leading up to finals week are a major stressor in students’ lives that is perceived by students as stress and is reflected in their HCC. In this scenario, HCC can be used as a measure of the effect of time off, indicating the disappearance of the stressor. Following this small pilot study, a significantly larger study with a significant number of participants, detailed stress questionnaires, and updated HCC measurement methodology and additional tests must be completed.
Funding
This work was supported by the Scholarly and Creative Activities Committee and the Provost’s Office of State University of New York College at Oswego and NIH 1R01ES023252-01A1 (PI-Gump BB).
Acknowledgement
We thank Ethan Walker for completing the confirmatory study of pooled finely-cut triple-extracted hair samples.
References
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010; 196: 32-37.
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013; 38: 1220-1235.
- Meyer JS, MA Novak. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012; 153: 4120-4127.
- Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. 2013; 23: 797-811.
- Kaushik A, Vasudev A, Arya SK, Pasha SK, Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens Bioelectron. 2014; 53: 499-512.
- Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol. 2015; 173: M1-10.
- Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. Evaluation of a method to measure long term cortisol levels. Steroids, 2011; 76: 1032-1036.
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013; 98: 2573-2580.
- Manenschijn L, Schaap L, van Schoor NM, van der Pas S, Peeters GM, Lips P, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013; 98: 2078-2083.
- Pereg D, Chan J, Russell E, Berlin T, Mosseri M, Seabrook JA, et al. Cortisol and testosterone in hair as biological markers of systolic heart failure. Psychoneuroendocrinology. 2013; 38: 2875-2882.
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010; 35: 1404-1409.
- Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011; 96: 1862-1865.
- Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012; 37: 611-617.
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress: Short communication. Stress. 2008; 11: 483-488.
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 2007; 30: 103-107.
- Karlén J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. Cortisol in hair measured in young adults - a biomarker of major life stressors? BMC Clin Pathol. 2011; 11: 12.
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013; 74: 639-646.
- Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology. 2011; 36: 1193-1200.
- Gidlow CJ, Randall J, Gillman J, Silk S, Jones MV. Hair cortisol and self-reported stress in healthy, working adults. Psychoneuroendocrinology. 2016; 63: 163-169.
- Faresjö A, Jullander M, Götmalm S, Theodorsson E. Higher perceived stress and poorer health reflected in elevated cortisol concentrations measured in extracts of hair from middle-aged healthy women. BMC Psychology. 2014; 2: 1.
- Gerber M, Jonsdottir IH, Kalak N, Elliot C, Pühse U, Holsboer-Trachsler E, et al. Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress. 2013; 16: 593-599.
- Andersen JP, Silver RC, Stewart B, Koperwas B, Kirschbaum C. Psychological and physiological responses following repeated peer death. PLoS One. 2013; 8: e75881.
- Gerber M, Kalak N, Elliot C, Holsboer-Trachsler E, Pühse U, Brand S. Both hair cortisol levels and perceived stress predict increased symptoms of depression: an exploratory study in young adults. Neuropsychobiology. 2013; 68: 100-109.
- Susman EJ, Schmeelk KH, Worrall BK, Granger DA, Ponirakis A, Chrousos GP. Corticotropin-releasing hormone and cortisol: longitudinal associations with depression and antisocial behavior in pregnant adolescents. J Am Acad Child Adolesc Psychiatry. 1999; 38: 460-467.
- D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011; 104: 348-353.
- Ng V, D Koh, SE Chia. Examination stress, salivary cortisol, and academic performance. Psychol Rep. 2003; 93: 1133-1134.
- Schiefelbein VL, EJ Susman. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. The Journal of Early Adolescence. 2006; 26: 397-413.
- Yang PJ, Lamb ME, Kappler G, Ahnert L. Children's diurnal cortisol activity during the first year of school. Applied Developmental Science. 2016; 21: 30-41.
- Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EF, van IJzendoorn MH. Children's hair cortisol as a biomarker of stress at school entry. Stress. 2013; 16: 711-715.
- Younge JO, Wester VL, van Rossum EF, Gotink RA, Wery MF, Utens EM, et al. Cortisol levels in scalp hair of patients with structural heart disease. Int J Cardiol. 2015; 184: 71-78.
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007; 30: 183-191.
- Slominski R, CR Rovnaghi, KJ Anand. Methodological Considerations for Hair Cortisol Measurements in Children. Ther Drug Monit. 2015; 37: 812-820.
- Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, et al. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016; 71: 12-18.
- Cooper GA, Kronstrand R, Kintz P, Society of Hair Testing. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci Int. 2012; 218: 20-24.
- Miller R, Plessow F, Rauh M, Gröschl M, Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013; 38: 50-57.
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2012; 37: 602-610.
- Kamarck TW, SB Manuck, JR Jennings. Social support reduces cardiovascular reactivity to psychological challenge: a laboratory model. Psychosomatic medicine. 1990; 52: 42-58.