
Special Article - Biomolecules
Austin Biochem. 2017; 2(1): 1009.
Chemometrics-Based Approach to Analysis of Phenolic Fingerprints of Red and Sugar Maple Bud Extracts
Meda NR1-3, Rott M1,2, Suwal S1,2, Poubelle PE3 and Stevanovic T1,2*
¹Centre de Recherche sur les Matériaux Renouvelables (CRMR), Département des sciences du bois et de la forêt, Université Laval, Canada
²Institut des Nutraceutiques et des Aliments Fonctionnels (INAF), Université Laval, Canada
³Centre de recherche en Rhumatologie et Immunologie, Centre de Recherche du CHU de Québec, Département de Médecine, Université Laval, Canada
*Corresponding author: Tatjana Stevanovic, Centre de Recherche sur les Matériaux Renouvelables, Département des sciences du bois et de la forêt, Faculté de foresterie, de géographie et de géomatique, Université Laval, 2425 rue de la Terrasse, Pavillon G-H Kruger, Québec, QC, G1V 0A6 Canada
Received: February 02, 2017; Accepted: March 14, 2017; Published: March 20, 2017
Abstract
We are reporting here the results of the first study on phenolic constituents of hot water and aqueous ethanol extracts of sugar (Acer saccharum Marsh) and red maple (Acer rubrum L.) buds. The bud extracts were compared for their phenolic fingerprints using Thin Layer Chromatography (TLC) supported by chemometric tools. The NP/PEG (Natural Products/Polyethylene Glycol) derivatized TLC plate was photographed using a digital camera. The image of each plate was subsequently processed using the Image J program (1.50a version). The global analysis of TLC tracks showed major differences in colours, intensities, and positions of spots on the phenolic fingerprint of the extracts of buds from red and sugar maple. The chemometric approach revealed that phenolic compounds with lower Retention factor (Rf) were revealed for sugar maple extracts, while those exhibiting bright intensity spots at Rf between 0.3 and 0.8 were observed for red maple extracts, thus indicating the differences between their phenolic fingerprints. The solvent used for extraction seems also to have an effect on the phenolic compounds extracted from maple buds. The results of the principal component analysis suggest that there are significant differences in the phenolic fingerprints between the bud extracts of sugar and red maple which are related to both the species and the solvent applied for extraction. The obtained results demonstrate the interest to apply TLC as an efficient method in screening of phytochemicals, especially in combination with new informatics and statistical tools.
Keywords: Acer saccharum; Acer rubrum; Buds; Phenolic constituents; Principal Component Analysis; TLC fingerprinting
Abbreviations
TLC: Thin layer chromatography; Rf: Retention factor, NP/ PEG: Natural Products/Polyethylene Glycol Reagent; PCA: Principal Component Analysis; RM: Red Maple; SM: Sugar Maple; HPTLC: High Performance Thin Layer Chromatography; HPLC: High Performance Liquid Chromatography
Introduction
Chemometrics is a branch of science that derives data by the application of mathematical and statistical methods for the extraction of useful information from physical and chemical phenomena [1]. Principal Component Analysis (PCA) is probably the most widespread multivariate chemometric technique employed to visualize and summarize large amount of data obtained from multivariate measurements in chemistry [2]. It reduces the dimensionality of the data by producing new linear combinations of the original variables while retaining the most significant information. Despite of their significant contribution in the analysis of phytochemicals, there is still the lack in their comprehensive use in many applications [3,4].
Phenolic compounds are secondary metabolites, widely distributed in the plant kingdom [5]. The interest in these substances was stimulated by their wide range of biological properties including antioxidant, anti-inflammatory, anticancer, and antimicrobial etc [6]. Also, the low incidence of several chronic diseases such as cardiovascular disease, diabetes, and cancer has been revealed to be in relationship with the intake of foods rich in polyphenols. So, plant polyphenolic compounds are becoming increasingly interesting for nutritionists as well as food and cosmetic industry professionals [7].
Sugar maple (Acer saccharum Marsh) and red maple (Acer rubrum L.), two important species of North-eastern American forests are notorious for their sap, which is used for maple syrup production. Traditional and anecdotal medicinal claims for other parts of these plants in Amerindian medicine have also incited the interest to study different maple tissues. Previous phytochemical studies have reported gallotannins, procyanidins, lignans, coumarins, and flavonoids in leaves, bark and wood of the red maple and sugar maple species [8- 11].
Up to now, one only study dealing about the involvement of phenolic compounds in the dormancy breaking of sugar maple bud has been reported [12]. However, buds contain mass of meristems that are undifferentiated embryonic tissues where regrowth of new tissues takes place after winter dormancy [13]. Bud extracts are also a category of plant products well known and widely used for gemmotherapy but also in homeopathy and in phytotherapy [14]. Tree buds are generally rich in plant growth hormones, microelements, vitamins, enzymes, free amino acids, polyphenols, nucleic acids and other bioactive compounds that are often found only in trace amounts in differentiated part of plants [15].
To the best of our knowledge this is the first study comparing phenolic fingerprints of water and ethanol extracts from sugar and red maple buds. So, the ability of these green solvents, to pick up significant and various phenolic compounds were assessed by semiquantitative analysis using Thin Layer Chromatography (TLC) supported by chemometric tools.
Materials and Methods
Chemicals
95 % ethanol was purchased from Commercial Alcohols Inc. (ON, Canada). Methanol, ethyl acetate, acetic acid were ACS certified and purchased from Fisher Scientific Chemicals, (Fair Lawn, NJ, USA). Kollisolv® PEG E 400 and 2-Aminoethyldiphenylborate for NP/PEG staining were supplied by Sigma Chemical Co. (St. Louis, MO, USA).
Plant materials
The dormant buds from SM and RM were harvested at the end of winter, from 10 to 24 March 2015. Voucher specimens (Acer saccharum Marsh, No. 176 and Acer rubrum L., No. 174) have been deposited at the herbarium of the Faculty of forestry, geomatics and geography (Faculté de foresterie géographie et géomatique) at Université Laval, Quebec City, Canada. The key morphological criteria described by Rouleau [16] were used to confirm their identities. Maple buds were collected from eight randomly selected vigorous trees (per species), pooled and mixed well before freeze-drying. Dried bud samples were then carefully crushed in a mortar to avoid the overheating and the powders were kept at -20°C until extraction.
Extraction procedure
Maple buds (10g) were extracted with 200 ml of solvent. The extraction with water was carried out using a water bath heated at 80°C under reflux conditions for 1 hour. The extraction with ethanol (95%) was performed by continuous shaking (230 rpm) at room temperature, for 24 h with an orbital shaker. The extracts were separated by filtration through Whatman No.3 filter paper (Whatman International Ltd., UK) in a Buchner funnel. Recovered solvent (ethanol) was evaporated under vacuum at 50°C using a rotary evaporator (Rotavapor® model R-215) until dryness, while aqueous extracts were firstly pre-concentrated under vacuum evaporator using same conditions and then freeze-dried.
Explorative analysis of phenolic fingerprints of maple bud extracts
TLC fingerprint processing: To provide a chemical fingerprint for each of the extracts studied, these were separated by TLC following by NP/PEG (Natural Products/Polyethylene Glycol Reagent) postchromatographic derivation. Analysis was performed on 20 cm X 20 cm flex TLC plates pre-coated with silica gel 60 F254 (Merck, Germany). Ten (10) μL of each extract (20 mg/mL) were deposited on the plate using a micro-syringe, at 10 mm from the bottom edge, for 15 mm wide bands, and spacing of 10 mm between the samples. TLC plates were then eluted for a distance of 120 mm in a pre-saturated chamber, by using 50 mL of mobile phase that consists of solvents mixtures; ethyl acetate: acetic acid: methanol in the ratio of 10/1/1 (v/v/v). After development, the plates were air-dried for 10 min and the spots were firstly visualized at daylight, and under UV light, at 254 and 366 nm without any derivatization. Phenolic compounds (flavonoids and phenolic acids) were then brought out by using NP/ PEG derivatization reagents.
Fingerprint analysis of phenolic content using chemometric tools: Chemometric tools were used to explore the phenolic compounds present in maple bud extracts by TLC fingerprinting according to a variant of the process described by Ristivojević et al. (2014) [17]. The NP/PEG post-derivatized TLC plate was photographed using a digital camera (Canon PowerShot ELPH 13015). The image of the plate was subsequently processed with the ImageJ program (1.50a version), a public domain Java-based image-processing program developed at the United States National Institute of Health. The image (1200 x 1500 pixels) was split into three filter channels: red, green, and blue, which are primary colour components (Image/Colours/Split channels). Each filter channel was then de-noised by applying the median filter function (Process/Filters/Median/Radius- 5 pixels) and subtracting background (Process/Subtract background…/Rolling ball radius- 50 pixels). At last, in order to change the images of tracks into chromatograms, a rectangular selection tool was used to outline the tracks (1200 x 125 pixels) and the line profile plots were achieved with plot profile option (Analyse /Plot Profile/ Selected) for each track according to the filter channel. The profile plot displays in twodimensional graph, the intensities of pixels (y-axis) by distance along the line.
A chromatogram (600 variables) was used as a dataset for chemometric analysis. Principal component analysis (PCA) was applied on the matrix resulted from the digitized chromatograms (4 samples × 600 variables) for each channel separately, in order to explore the fingerprints data by extracting important information and by finding out the main sources of variability. The analysis was performed with SAS software package (SAS Institute Inc., Cary, NC, USA).
Results and Discussion
The Thin Layer Chromatography (TLC) following by NP/PEG reagent (Diphenylborinic Acid 2-aminoethyl Ester/ Polyethylene Glycol) was used to highlight phenolic components in the maple bud extracts. This reagent reacts especially with flavonoids and phenolic acids through their phenolic hydroxyl groups to give a fluorescence quenching compound under UV radiation lamp at 366 nm [18].
Visual examination of the image capturing the TLC chromatograms (Figure 1A) revealed differences in phenolic chemical composition between the maple bud extracts. First, it appeared that ethanolic extract provided more spots on their tracks for all samples (10 spots for RM and 11 spots for SM) than hot water extracts (9 spots for each sample track). But the blatant remark of these fingerprints was the brighter intensity of spots for the extracts of Red Maple buds (RM) compared to those of the Sugar Maple (SM). These findings suggest a higher concentration of phenolic compounds in the extract of red maple buds. The global analysis of tracks showed differences in colors, intensities, and positions of spots that reflect a significant difference in the chemicals fingerprint of these samples. The subjectivity of the TLC analysis by visual examination could be counterbalanced by chemometric tools for the fingerprints analysis [3].
The chemometric techniques allow to compare and explore complex and multivariate data to recover the best information in order to find the relationship between the samples and variables in a given dataset [2]. Despite of their significant contribution in the analysis of phytochemicals, there is still a lack of their comprehensive use in many applications [3,19].
The image of the TLC plate was therefore processed by ImageJ software to generate the line profile plots of chromatograms for each track in order to perform full chemometric processing analysis. Red (R), Green (G), and Blue (B) filter channels were applied to improve the selectivity by differentiating compounds (spots) according to their fluorescence colours (Figure 1B). The variables (corresponding to the relative retention factor along each migration track) of each of the samples digitized chromatograms were subjected to the most often used unsupervised method, namely the Principal Component Analysis (PCA).
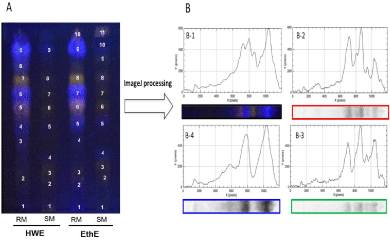
Figure 1: TLC fingerprinting of extracts from red and sugar maple buds.
A. TLC fingerprint of phenolic compounds presents in maples bud samples. Hot water Extract (HWE) and Ethanol Extract (EthE) of red maple (RM) and sugar
maple (SM). Post derivatizations were then performed with NP reagent (visualized at UV 366 nm for plate).
B. ImageJ processing used to generate in two dimensions graph, the chromatographic plot of hot water extract of red maple buds (B-1) according Red (B-2), Green
(B-3) and Blue (B-4) channels.
PCA is a powerful tool for identifying patterns in high dimensional data and for emphasizing differences and similarities among studied samples. To do that, it condenses the main sources of the data’s variability by producing new linear combinations of the original variables, called Principal Components (PCs), while retaining the large and most significant proportion of information. Called scores plot, the graphical representation of samples in the reduced space composed by these new PCs (often in two or three dimensional) provides useful information from the data set and contributes to a better visualization [20,21].
The explorative analysis revealed that, even if for each filter channel, the first three Principal Components (PCs) regroup all sources of variability (Scree Plot of each channel in complementary data), the blue channel filter favors easier viewing of differences between sugar and red maple bud samples. Based on the coordinates of the samples Principal Component (PC), Three Dimensional (3D) visualizations of the distribution relative to each other were generated to describe the optimal view of their differences. This 3D scatter plot of the PCA is shown in (Figure 2A) in which the dotted lines represent samples arranged behind the plane while solid lines display samples placed inside or ahead of the plane. The most important component (PC1) represented up to 65.9 % of the total variance clearly allowed to differentiate the red maple bud extracts (samples 1 and 3 for hot water and ethanol extraction, respectively, that display positive coordinates on this PC 1) from all the sugar maple bud extracts. These findings suggest a real interesting way of discriminating between the phenolic constituents of bud extracts from two maple species. The second component PC 2 with 26.5 % of the total variance allowed to discriminate samples from hot water and ethanol extraction, for red maple buds. The last component PC 3 distinguished sample 2 to sample 4 that were respectively, the hot water extract and the ethanol extract of sugar maple buds.
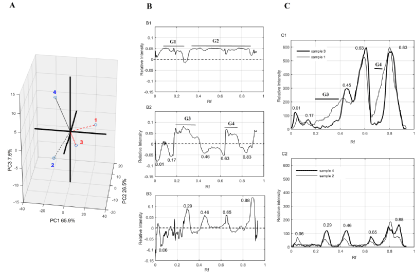
Figure 2: Chemometric analysis of phenolic fingerprint of extracts from red and sugar maple buds.
A. Scatter plot of the Three Principal Components (PC) extracted from PCA analysis according to blue channel filters.
B. Loadings plot for the PC1 (B-1), PC2 (B-2) and PC3 (B-3) for blue channel filters; Rf (Retention factor), G character followed by number appoint group of TLC
spots.
C. The line profile plots of chromatograms of maple bud extracts.
The loading plots according to each component were displayed in order to identify variables (the Rf values of each compound/spot on TLC plate) that contributed to the greatest variation among different samples tested (Figure 2B). So, for PC1 (Figure 2-B1), two groups of TLC spots seemed to have most contribution to the discrimination between the samples. The first group (G1) contains spots having a Retention factor (Rf) lower than 0.3 whereas the second group (G2) represents the spots with a Rf between 0.3 and 0.8. Visual examination of the TLC plate further confirmed that it could be separated into two parts: the first part that gathers compounds with low Rf, seems to be more important in sugar maple bud extracts, thus would correspond to the G1. The second part of the TLC plate, which could be so far the most obvious variation assimilated to the brightest intensity spots of red maple bud extracts that fits to the G2. In sum, phenolic compounds with lower Rf values determined in sugar maple bud extracts and those having Rf values between 0.3 and 0.8, characterized by bright intensity spots, in red maple bud extracts, could have strongly contributed to the differences observed between the TLC fingerprints of the two species studied.
The loading plot for PC2 (Figure 2-B2) revealed that spots with Rf values of 0.01, 0.15, 0.26 (G3), 0.46, 0.63, 0.70 (G4) and 0.83 have the most impact on this PC that discriminates the hot water and ethanol extract fingerprints for red maple buds. In a similar way TLC bands with Rf values of 0.06, 0.29, 0.46, 0.65 and 0.88 appear to be responsible for the differences observed for fingerprint of sugar maple bud extracts related to the extraction solvent (Figure 2-B3). By close examination of the line profile plots of chromatograms for these samples for blue filter (Figure 2C), it appears that the variables exposed by chemometric analysis, are really at the root of the observed differences of the phenolic fingerprints of samples.
Fingerprinting analysis of complex mixtures such as plant extracts have often been performed by HPTLC (High Performance Thin Layer Chromatography) or HPLC (High Performance Liquid Chromatography) that provides unparalleled performance in chemical separations comparatively to TLC [22]. However, careful spotting of samples could lead to conclusive results for chemometric analysis by TLC. The results obtained in this research indicate that despite the low capacity of separation compared to the more advanced analytical chromatographic techniques, TLC displays advantages to be easy, fast, inexpensive, and allowing screening of a large range of phytochemicals. Furthermore, under optimum separation conditions, this screening technique provides a chemical fingerprint of a sample, while providing the first approach to the investigation of the chemical composition of plants for the edification of monographs in most pharmacopoeias [23]. Based on the results of this study, the use of chemometric tools gave a powerful and successful contribution to the analysis of the fingerprints of maple buds extracts. Because of their contribution to the authentication of the sugar and red maple bud extracts, the compounds revealed by our study will have to be further identified as chemical markers of these extracts and species they originate from.
Conclusion
We are presenting for the first time, the results on the comparison of the phenolic fingerprint of hot water and ethanol extractions of sugar and red maple buds. The TLC fingerprint analyses revealed that sugar and red maple bud extracts displayed considerable differences. Phenolic compounds with lower Retention factor (Rf) point out in to the sugar maple samples and those characterized by bright intensity spots at Rf between 0.3 and 0.8 for red maple sample could strongly contribute to differentiate of these species. Also, solvent used for extraction seems to have an effect on the phenolic content of extracts from maple buds. Quantitative assays on the phenolic constituents and the assessment of the antioxidant capacity of these bud extracts, which make a part of the ongoing research, might confirm the interest of the maple bud extracts and/or their active ingredients.
Acknowledgment
The authors are grateful for the financial support from the Conseil de recherches en sciences naturelles et en génie du Canada (CRSNG) and the Décacer and Levaco Inc. The authors are grateful to Mr. Clermont Levasseur from Decacer Inc. for his personal involvement during sampling of maple buds. We would like to thank Mr. Yves Bédard, Sara Hattab and Alex Simard for technical assistance, and Mme Helène Crépeau for statistical model validation.
References
- Singh I, Juneja P, Kaur B, Kumar P. Pharmaceutical applications of chemometric techniques. Int Sch Res Not. 2013.
- Kumar N, Bansal A, Sarma GS, Rawal RK. Chemometrics tools used in analytical chemistry: An overview. Talanta. 2014; 123: 186-199.
- Komsta L. Chemometrics in fingerprinting by means of thin layer chromatography. Chromatogr Res Int. 2012; 2012: 1-5.
- Gemperline PJ. Introduction to Chemometrics. In: Practical Guide to Chemometrics. Second Edition. CRC Press. 2006; 1-6.
- Abdi K, Safarian S, Esmaeili N, Ebrahimzadeh H. Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacogn Mag. 2011; 7: 74-80.
- Sankhalkar Ss, Vernekar V. Quantitative and Qualitative analysis of Phenolic and Flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacogn Res. 2016; 8: 16-21.
- Royer M, Diouf PN, Stevanovic T. Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem Toxicol. 2011; 49: 2180-2188.
- González-Sarrías A, Li L, Seeram NP. Effects of Maple (Acer) Plant Part Extracts on Proliferation, Apoptosis and Cell Cycle Arrest of Human Tumorigenic and Non-tumorigenic Colon Cells. Phytother Res. 2012; 26: 995-1002.
- Wan C, Yuan T, Li L, Kandhi V, Cech NB, Xie M, et al. Maplexins, new a-glucosidase inhibitors from red maple (Acer rubrum) stems. Bioorg Med Chem Lett. 2012; 22: 597-600.
- Yuan T, Wan C, González-Sarrías A, Kandhi V, Cech NB, Seeram NP. Phenolic Glycosides from Sugar Maple (Acer saccharum) Bark. J Nat Prod. 2011; 74: 2472-2476.
- Yuan T, Wan C, Liu K, Seeram NP. New maplexins F-I and phenolic glycosides from red maple (Acer rubrum) bark. Tetrahedron. 2012; 68: 959- 964.
- Thakur ML. Phenolic growth inhibitors isolated from dormant buds of sugar maple (Acer saccharum Marsh). J Exp Bot. 1977; 28: 795-803.
- Horvath DP. Bud Dormancy and Growth. In: Plant Developmental Biology - Biotechnological Perspectives: Volume 1. Verlag Berlin Heidelberg. Springer. 2010.
- Ieri F, Innocenti M, Possieri L, Gallori S, Mulinacci N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J Pharm Biomed Anal. 2015; 115: 1-9.
- Donno D, Beccaro GL, Cerutti AK, Mellano MG, Bounous G. Bud Extracts as New Phytochemical Source for Herbal Preparations-Quality Control and Standardization by Analytical Fingerprint. Rao AV, Rao LG, editors. In: Phytochemicals - Isolation, Characterisation and Role in Human Health. InTech. 2015; 187-218.
- Rouleau R. Petite flore forestière du Québec. Québec; Montréal: France- Amérique. 1979.
- Ristivojević P, Andrić FL, Trifković JĐ, Vovk I, Stanisavljević LŽ, Tešić ŽL, et al. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts: Classification of propolis. J Chemom. 2014; 28: 301-310.
- Brasseur T, Angenot L. Le mélange diphénylborate d’aminoéthanol-PEG 400: Un intéressant réactif de révélation des flavono´des. J Chromatogr. 1986; 351: 351-355.
- Gemperline P. Practical Guide to Chemometrics, Second Edition. Boca Raton, Florida: CRC Press. 2006.
- Brereton RG. Pattern Recognition. In: Chemometrics: Data Analysis for the Laboratory and Chemical Plant. John Wiley & Sons, Ltd. 2003; 183-269.
- Ren S, Hinzman AA, Kang EL, Szczesniak RD, Lu LJ. Computational and statistical analysis of metabolomics data. Metabolomics. 2015; 11: 1492- 1513.
- Zheleva-Dimitrova D, Balabanova V, Gevrenova R, Doichinova I, Vitkova A. Chemometrics-based approach in analysis of Arnicae flos. Pharmacogn Mag. 2015; 11: 538-544.
- Skalicka-Woniak K, Gowniak K, Widelski J. Plant Materials in Modern Pharmacy and Methods of Their Investigations. In: Thin Layer Chromatography in Phytochemistry. CRC Press. 2008; 16-35.