
Review Article
Austin Biochem. 2017; 2(2): 1012.
Phytoremediation: A Green Technology to Clean Up the Sites with Low and Moderate Level of Heavy Metals
Singh H¹*, Verma A², Kumar M³, Sharma R¹, Gupta R4, Kaur M5, Negi M¹ and Sharma SK¹
¹Ecology, Climate Change and Forest Influence Division, Forest Research Institute, India
²Sardarkrushinagar Dantiwada Agricultural University, India
³Forest Informatics Division, Forest Research Institute, India
4Department of Biology, Fiji National University, Fiji Islands
5Plant Physiology Discipline, Forest Botany Division, Forest Research Institute, India
*Corresponding author: Hukum Singh, Climate Change and Forest Influence Division, Forest Research Institute, Dehradun- 248 006, India
Received: July 27, 2017; Accepted: September 04, 2017; Published: September 11, 2017
Abstract
Contamination of soil and water with heavy metals is a serious concern worldwide. It adversely affects - plant growth as well as human health. Remdiation of these contaminated soils and water using phytoremediation provides an opportunity to regain the prestine state of the soil and water environment. This review discusses about the cost effective phytoremediation technology that can be used to remove heavy metalsand other pollutants from contaminated soil and water. Plants having hyperaccumulators use their internal complex system of highly effective homeostatic mechanisms to control the uptake, accumulation, trafficking, and detoxification of metals. A concise overview of numerous approaches of phytoremediation in this review demonstrates that despite of some limitations, phytoremediation is an effective approach for the removal of heavy metals and other contaminants from soil and water.
Keywords: Phytoremediation; heavy metals; Vacuolar sequestration; Phytofilteration; Phytovolatisation
Introduction
Heavy metals are found in soil and water naturally and are generally released from various natural and anthropogenic sources [1] (Figure 1). Heavy metals disrupt nutrients and water uptake, affect production of Reactive Oxygen Species (ROS), reduce photosynthetic efficiency, alter cell division, alter nitrogen metabolism and thus affect plant growth [2]. Continuous uptake of heavy metals in humans through contaminated foods can cause oxidative stress by over production of ROS, upper gastrointestinal cancer and many immunological syndrome including carcinogenic effects, teratogenesis and mutagenesis [3].

Figure 1: Sources and effects of HMs.
Three different remediate processes i.e. biological, physical and chemical are used to cleanup heavy metal contaminated soils. Bioremediation is a process in which living organisms are used to remove contamination to re-establish the natural conditions. Generally microorganisms and plants are used to detoxify heavy metals from soil. Other bioremediation techniques such as bioventing, bioleaching, bioreaction, bioaugmentation, rhizofiltration and biostimulation are also helpful to remove heavy metal toxicity. Bioremediation is one of most ecofriendly, cost effective option to rectify soil contaminants. Bioremediation agents includes both microorganisms and plants which detoxify heavy metals in soil and water.. Phytoremediation is an alternative cost effective, ecofriendly, non-invasive, green technology to clean up or tackle the sites with low and moderate level of heavy metals [4].
Hyperaccumulator plants (Table 1) have potential to absorb heavy metals by 50 to500 times more than the normal plants [5]. This is an evolutionary adaptation of plants in unfavorable habitats with high concentration of metals in soils or rocks. But the advantages of hyperaccumulation of metals in plants remain unknown. Ideally hyperaccumulator plants have extensive root systems, and tolerate to a high concentration of metal pollutants. In recent years, different genetic engineering approaches have been used to increase production and productivity of hyperaccumulator plants. In combination with conventional agronomic practices and genetic engineering, the heavy metal absorption by plant can be enhanced [6]. Therefore there is an urgent need to investigate both conventional and genetically modified potential hyperaccumulators, which can be planted in contaminated sites to remove heavy metals in sustainable way to maintain environmental quality of the ecosystems. This review presents recent progresses, challenges and future prospects in the field of phytoremediation.
S.No.
Type of Plant
Resistance strategy to Heavy Metal Toxicity
1.
HM sensitive plants
Plants which do not show resistance to HM toxicity and their biochemical machinery shatters in response to increased HM soil/water concentration.
2.
HM resistant plants
Plants which have different mechanisms to prevent the HM accumulation inside the cells by either active transport mechanism or through restriction of HM transport from soil to root system. They are also termed as “Excluders”
3.
Hyperaccumulators
Plants which have unique mechanism to deal with HM toxicity by actively taking up HMs from soil/water and accumulate in their aboveground parts. They are pioneering research subject for the emerging technology “Phytomining”
Table 1: Classification of Plants on the basis of HM toxicity.
Heavy Metals (HMs): Origin and Effects
Heavy metals accumulate in the soil either from natural process of earth crust or anthropogenic sources. In the recent decades an annual worldwide release of heavy metals reached 22,000 metric ton for cadmium, 939,000 metric ton for copper, 783,000 metric ton for lead and 1,350,000 metric ton for zinc due to different human activities [7]. The cost of cleaning up contaminated sites through conventional approaches is often very high. In the USA alone, US $ 6-8 billion is annually spent in remediation efforts, with global costs in the range of US $ 25-50 billion [8,9]. The largest segment in this market is the clean-up of different sites contaminated with radionuclides as a result of nuclear weapon preparations during the Cold War [10]. According to the European Environmental Agency (EEA), it is estimated that, in Europe, potentially polluting activities have occurred at about 3 million sites, of which, >8% (or nearly 250 000 sites) are highly contaminated and projected upto>50% by 2025. In the Europe, the annual cost of soil degradation alone is estimated at some $38 billion [11].
Recently high Arsenic (As) content is reported in South Asian countries because of presence of (As)-containing rocks on parent material in this area. The most significant anthropogenic sources are mining, industrial discharge, sewage effluents, pesticides, fertilizers and bio-solids in agriculture [12]. Heavy metals are difficult to remove from soil because unlike organic substances they do not degrade into small molecules and therefore keep accumulating into the environment. Heavy metals are those trace elements having an atomic density greater than 5 gm cm-3.
Heavy metals viz. Co, Cr, Cu, Mn, Mo, and Zn are essential in the form of micro nutrient growth and development of both plants and animals, but high concentration causes toxicity. Toxic heavy metals i.e. As, Hg, Cd, Pb, Cu, Zn, Sn and Cr could show adverse effect to human health and plant performance. Out of these Cu and Zn are essential heavy metals, agriculture productivity in many parts of the world is limited by deficiency of these nutrients [13]. Toxicity level of Hg, As, Cd, Pb, Zn, Cr are usually unaltered by all biochemical pathways and thus remain tend to accumulate in soil and aquatic elements [14]. Heavy metals deserve a special attention to study.
Heavy metals enter in food chain and reach to different trophic levels causing bioaccumulation. In human body, heavy metals induce severe health effect like kidney damage, osteoporosis, increased blood pressure, failure of reproductive system and liver disorder. Heavy metals (Pb and Cd) induce carcinogenesis, teratogenesis and mutagenesis in human beings [1]. In plant Cu is essential micronutrient element for growth and development but intracellular free Cu ions in excess produce ROS by auto-oxidation and Fenton reaction [15,16]. Also, hydroxyl radicals react to cause membrane lipid peroxidation, cleavage of the sugar phosphate backbone of nucleic acids, and protein denaturation. In addition, Cu can displace other divalent cations coordinated with macromolecules, causing their inactivation or malfunction [17]. Zn is also an essential nutrient for plants which acts as a co-factor required for the structure and function of numerous enzymes [18] energy production and structural integrity of membranes [19]. High levels of Zn inhibit many plant metabolic functions resulting in slow growth. Zinc toxicity, limits the growth of both roots and shoots and produces leaf chlorosis. Even though it is not redox active, higher levels of concentration are toxic because it can displace other metals (e.g. Fe, Mn and Cu) in the cell [20,21]. Physical and chemical methods of remediation have limitations because of its high cost, intensive labor and production of secondary pollutants. Therefore, bioremediation is a viable option to remediate heavy metal contaminated soils in ecofriendly and cost effective manner.
Phytoremediation
Phytoremediation (phyto, meaning “plant”, and remedium, meaning “restoring balance”) is a cleanup green technology, which involves the use of plants and their associated rhizospheric microbes for treating environmental contaminants such as Heavy Metals (HMs), organic compounds or radioactive elements, in soil, groundwater or industrial wastes. General advantage of phytoremediation is noninvasive, ecofriendly, energy efficient and cheaper than other methods like soil excavation, soil washing, burning and the possibility of metal recycling [4-22]. Phytoremediator plants have some basic characteristics as given by Punshon and Dickinson [23], summarized in (Figure 2) with economically viable secondary use e.g., energy from biomass, pulp and paper production [24]. Plant age, environment factors, microbial colonization, size of the metals and translocation to different parts are the crucial factors that affect the uptake of HMs to the plants. Some tree species like Populus and Salix sp., have shown to meet all of these requirements as reported by many workers [25- 28]. These trees have a high biomass production, extensive roots, high rates of transpiration and easy propagation. At the same time, several studies have revealed a remarkable clonal variability in their ability to accumulate/tolerate HMs [29-32]. Phytoremediation takes advantage of the fact that a living plant can be considered a solar-driven pump; which can extract and accumulate elements from the environment [33].
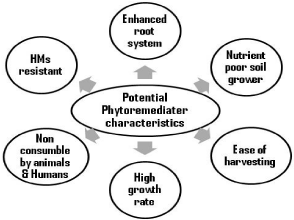
Figure 2: Basic characteristics of a phytoremediater plant.
Phytoremediation: Mechanism
Plants exhibit different homeostatic mechanisms for coping with excess metals. These mechanisms determine the amount of metal uptake by the plants. Metal uptake in roots can be regulated by the exudation of organic acid ions, the binding effect of the cell wall and the flux of the ions through plasmalemma metal transporter proteins. In cytoplasm, metals are chelated and transported towards organelles by peptidicchelators. Also excess of metallic ions can be directed to vacuole or apoplast by membrane transporters. Metals are mobilized through the xylem from roots to aerial structures in a process driven by transpiration. Inside leaf cells, a regulated network of membrane transporters and chelators directs metals to their final destination. A further defense line against metal induced ROS involves enzymes and reducing metabolites. Response to metal stress also includes expression of general defense proteins and signaling elements as such as calcium and ethylene (Figure 3).
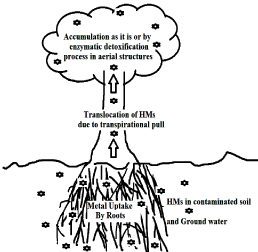
Figure 3: Phytoremediation Mechanism: Main steps of the phytoremediation
process occuring in a phytoremediater species.
Root uptake
First stage of Heavy metals uptake involves ion absorption from the soil, and distribute into the root cells. There are several compounds that can perform the function of transportation and accumulation of HMs in tissues and other locations like metal ligands e.g. Organic Acids (OAs) such as citrate, malate, oxalate [34]. OAs also play an alternate role of excluding metals from plants [35]. In wheat, Al uptake is inhibited by the exudation of OAs which forms the OA-Al complex [36,37]. Similarly in case of P. tremula root, Copper (Cu) uptake is inhibited by the exudation of formate, malate and oxalate, while Zinc (Zn) uptake is inhibited by exudation of formate [38]. Siderophores like mugineic acids and avenic acids are released by some plant species to enhance the bioavailability of HMs from soil for root uptake as reported in grass species [39].
Vacuolar sequestration
After uptake from root, HMs are usually sequestered in the vacuoles of plant cell. Through transporter protein mainly ZIP (zinc/iron-regulated transporters) family members, HMs enter the cytosol which further stimulates Phytochelatin Synthase (PCS) - enzyme which catalyzes the synthesis of Phytochelatins (PCs) from glutathione. HM phytochelatin complexes are low molecular weight complex which are transported to vacuole via tonoplastlocalized ATP-Binding-Cassette (ABC) transporters. HMs are also sequestered in the vacuole by tonoplast-localized Cation/Proton Exchanger (CAX) transporters which direct exchanges of the HMs with protons. In the vacuole, Low Molecular Weight (LMW) HM PC complex accumulates into High-Molecular-Weight (HMW) complex with more HMs. HMs may enter the vacuole via a direct exchange mechanism of different HM-protons exchanger transporters like Metal Tolerance Protein (MTPs) and Natural Resistance Associated Macrophage Protein (NRAMPs) (Figure 4). These transporter proteins reside in the tonoplast and mediate passage of metal ions for compartmentation or remobilization [40].
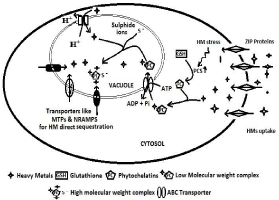
Figure 4: HMs root uptake and Vacuolar Sequestration by phytochelatins
and other tonoplast bound transporters.
Metal uptake enhancement
Mycorrhiza is a symbiotic association of fungi with the plants, protects from heavy metal pollution by binding them into cellwall components or by storing high amounts of HMs in cytosol. Mycorrhizae also produce growth-stimulating substances for plants, hence encouraging mineral nutrition and increased growth and biomass necessary for phytoremediation [41].
Smeeth and Reed [42] believed that mycorrhiza can be found in those plants which are grown in natural conditions. Mycorrhizae can play a crucial role in protecting plant roots from heavy metals as reported by Galli et al. [43]. But their efficiency of protection varies with species to species and type of heavy metals and its mycorrhizal. The extrametrical fungal hyphae can extend deep into the soil and uptake large amounts of nutrients, including heavy metals, to the host root spores of arbuscular mycorrhizal fungal taxa such as Glomus and Gigaspora were reported by many workers [44-47]. Pawlowska et al, recovered spores of Glomusaggregatum, G. fasciculatum and Entrophospora sp. from the mycorrhizospheres of the plants while studying a calamine soil rich in Cd, Pb and Zn at Poland [48]. Turnau studied the localization of heavy metals within the fungal mycelium and mycorrhizal roots of Euphorbia cyparissias from Zn contaminated wastes and found higher concentrations of Zn deposited within the fungal mycelium and cortical cells of mycorrhizal roots [49]. It was found that arbuscular mycorrhiza fungus can transport Cd from soil to subterranean clover plants growing in compartmented pots but that transfer is restricted due to fungal compartmentalization [50]. Many reports confirmed the increase in heavy metal level in plants due to arbuscularmycorrhiza [47,50,51].
Metal compartmentalization
At the cellular level, cell walls bind to the metal ions assisting them towards cytoplasm by cationic exchange [52]. Metals can bind to either pectin [53] or proteins as oxalate oxidase [54]. Metals can diffuse into the apoplast of some root cells but its transport is blocked by the impermeable casparian strip in the endodermal layer. At this point, plants have a series of metal transporters involved in metal uptake and homeostasis, which regulates its movement toward the symplast and subsequent loading into the vascular tissues [55].
Now, in the plasmodesmata, metals are transported by Heavy Metal ATPases (HMAs) [56], Zrt-Irt- related proteins (ZIP) [57,58], COPT-type transporters [7] and cationantiporters [59]. Cd and Zn are chemically very similar indicating a similar uptake and transport pathway [60].
Glutathione (GSH) and Phytochelatins (PCs)
In HM toxicity glutathione acts as ligand for their sequestration and releiving the oxidative stress caused due to them. Researchers reported increase in the reduced form of GSH upto 30 folds against Cd toxicity in Phragmitesaustralis [61]. However, in some reports no such incremented GSH synthesis was observed which can conclude that glutathione has no direct role in HM detoxification and it acts through the formation of Phytochelatins [62,63]. Phytochelatins has been reported in the detoxification of HMs in plants as well as in other organisms, where they acts as ligand to bind with these HMS to form complexes which is signalled further for compartmentalization. Actually the term “Phytochelatin” is a misnomer as they occur in microorganisms also. Apartly they are also reported to be found in nematode C. elegans, slime molds Dictyostelium, acquatic midge Chironomus oppositus as reviewed by [64].
Phytochelatins classified as Class III MTs are polypeptides with Glu-Cys dipeptide followed by a terminal Gly. (-Glu-Cys)- n-Gly, where n>2, present in several plants and microorganisms and resembles structurally to glutathione (GSH; -Glu-Cys-Gly), synthesized by the enzyme phytochelatin synthase which is activated by HM ions and results in the vacuolar sequestration of HMs and also have role in the homeostasis of essential metal ion metabolism [64].
Metallothioneins (MTs)
(MTs) are low molecular mass, cysteine-rich, metal binding proteins that are used for HM detoxification by intracellular sequestration [65]. These chelators bind to the metals and form a complex that are transported to vacuole e.g. Zn is transported into the vacuole by MTPs (metal tolerance protein). MTP1 and MTP3 localize at the vacuolar membrane [66,67] and are expressed to make the plant Zn tolerant [68,66]. In the case of Cd, AtHMA3 plays a role in its accumulation in vacuole [69,70]. For Cu, transporters such as PAA1 (HMA6), PAA2 (HMA8) and HMA1 are critical for transportation of Cu into plastocyanin in the chloroplast [71]. Cu can also be transported into the mitochondria when it enters the respiratory electron transport chain. Then intracellular distribution of metals is done by chaperons. Metal chaperons associate with ATPases in detoxification of HM in roots [72].
Metal Translocation to Shoots and Shoot Metabolism
HMs are transported from roots to epidermal tissue, then to the pericycle or xylem parenchyma, and finally loaded into the xylem through transmembrane [55]. In Arabidopsis, ATPases HMA2 and HMA4 are responsible for transporting and accumulating Zn from roots to shoots [73,74], ATPase HMA5 is involved in Cu transportation [75]. Translocation of metals also involves few amino and organic acids e.g. Cd, Cu and Zn require citrate, malate, histidine, nicotinamineetc [20]. In shoots, excess HMs can cause oxidative stress and damage to the exposed cells by replacement of metal ions in pigment and other essential molecules like chlorophyll. Many of the photosystem components got affected due to HM toxicity which disturbs the photosynthesis (Figure 5). The redox active metals (Cu) and non-redox active metals (Cd and Zn) both can cause oxidative damage. To prevent the cells from this damage, plants have an inbuilt antioxidative defense system based on reducing metabolites (GSH) and enzymes (peroxidase) that regulates the redox state. Glutathione is a fundamental molecule that is synthesized from Glu, Cys and Gly by glutamylcysteinesynthetase and GSH synthetase. Glutathione is a precursor of PCs. It can bind to metals and metalloids, and eliminates reactive oxygen radicals induced by HM in cells and maintains redox homeostasis for metabolism, signal transduction and gene expression [76]. A heavy metal, strontium is a dangerous and inhibits the growth and development of plant, the response of Phaseolus mungo to strontium concentration was studied by [77].
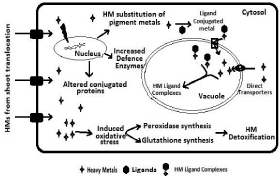
Figure 5: HM accumulation and other processes of detoxification that takes
place in leaf cell.
Classification of Phytoremediation Process
Phytoremediation has been categorized into phytoextraction, phytofilteration, phytostablization, phytovolatisation and phytodegredation.
Phytoextraction
Phytoextraction is also known as phytoaccumulation, which is used to uptake contamination from soil and water by roots to translocate contaminants to the shoot and leaves [78]. Hyperaccumulation plants absorb heavy metals in 50-500 times higher than non hyperaccumulation plants without any adverse effect on growth and development [79]. These plants are generally small and slow growing and often rare species of limited population size and restricted distribution in ecosystem [80].
Currently, more than 450 plant species of 45 families have been identified as a hyperaccumulator, which represents less than 0.2% of all angiosperms [1]. These plants are mainly included from Brassicaceae, Asteraceae, Fabaceae, Lamiaceae, Poaceae, Euphorbiaceae, Caryophyllaceae and Violaceae [6,81]. Recently it has been reported that unlike normal plants, wild huperaccumulators proliferate roots positively in patches of high metal availability [82]. Different species of British Thlaspi caerulescens were used as Zn hyperaccumulator but high uptake of Cd, Co, Mn and Ni has also been reported with same mechanism of absorption and transportation [83]. More than one metal accumulator also observed in many other species of Brassicaceaesuch as Brassica, juncea, Brassica napus, Crassulaceaeie and Sedum alfredi.
Some hyperaccumulators are more resilient to higher Nickel (Ni) concentration; Ni enters xylem of roots and transport rapidly to the shoot xylem along with more effective transpiration. Brassicacoddii from Central Africa is highly tolerated to Ni in the soil environment. It effectively absorbs and translocates into shoot and mainly concentrates in leaves [79]. Cobalt (Co) is also accumulated by Brassicacaddii in both either presence or absence of Ni, Co inhibits Ni absorption. Some tree species i.e. Populus species and Salix species are extensively used for Zn and Cd accumulation from contaminated soil.
In recent days, different genetic engineering approaches have been used to enhance ability of hyperaccumulators. Transporter protein like CDF, ZIP, IRTP are highly correlated with accumulators of heavy metals in different plant species [2]. Transgenic Nicotianatobaccum increase Cd uptake and tolerance, Pb in Nicotianaglauca, Zn in Lactuca sativa and Branicaoleraea and As in Arabidopsis thaliana [84].
Phytofiltration
Phytofiltration is a technique to remove impurities from ground water and contaminated waste water by plants. For filtration different plant parts are used, roots (rhizofiltration), seedlings (blastofiltration) and excised plant shoots (caulofiltration. Phytofilterates can be aquatic, semi aquatic and terrestrial, slow growth and efficient metal binding capacity [85]. Plants grown in hydroponic are more efficient in rhizofiltration to absorb contaminants than typical water plants.
Callitrichecophocena effectively treats water contaminated with Thallium (Tl), Cd, Zn and Pb [86], Juncusacatus is used for Crcontaminated ground water [87] and Plectranthusamboinics shows tolerance to a wide range of Pb [88]. Two aquatic macrophytes, Pistiastratiotes and Azolla pinnate were found to remove Hg contamination from coal mining effluents. Cladophora an alga was used to treat Arsenic contaminated water [89]. Some terrestrial plants like sunflower, Brassica junecea are also used to remove heavy metals contaminate from water [85].
Phytostabilization
Phytostabilization is also called as phytosequestration/ phytodeposition which deals with fixing/sequestering pollutants in soil near the root but not in plant tissues that prevents heavy metal migration to either ground water or in food web. Recently two grasses i.e. Agrostis species and Festuca species were used in phytostabilization of Cu, Zn, Pb contaminated soil from Europe, China and America. Combination of grass and tree co-cultivation shows potential to photostabilization. Tree plantation can be used to reduce soil erosion, prevent water erosion, immobilize the pollutants by accumulation and provide a space around the roots where pollutants are fixed or stabilized. For example spruce (Piceaabien) roots minimize phytostabilization capacity because trace elements such as Cd, Cu, Pb and Zn are absorbed by their roots. Therefore phytostabilization is different from other approaches; unlike other it is not a permanent solution and is used mainly to limit movements of HMs. It is a management strategy to inactivate toxics.
Phytovolatization
Phytovolatization is also known as phytoevaporation in which plants uptake volatile organic pollutants and some heavy metals like Hg, Se and As from soil. It’s a controversial technique, limits the complete removal of pollutants from soil only transferred from one segment (soil) to another (atmosphere) from where it can redeposit. Brassica juncea is used to remove Se from soil [32,90]. Se converts to volatile methyl sclenate and is removed [91,92]. Astragalusbiscularts is also used for Se evaporation in which Se converts into methyl selenocynitive by using selenocysteine methyltrasferare enzyme [93]. In Pterisvittata as was effectively evaporated in the form of arsenite/arsenate. Transgenic Arabdopsis and Tobacco plants are also engineered with bacterial genes (merA and merB) (mercury reductase) which can volatize Hg almost 10-100 times more Hg than wild plants [94].
Phytostimulation
Phytostimulation is also called rhizodegradation in which microorganisms are used to breakdown organic pollutants in the soil. Microorganisms enhance metal availability and mobility in soil that helps plants to grow well even under metal strain condition. Bacteria, such as Bacillus mucilaginosus (K-soluble), Bacillus megaterion (P-soluble) and Azotobacter chroococcum (Nitrogen-fixing) can help plant growth in several ways i.e. lowering pH, producing plant growth regulations such as Indoleacetic Acid (IAA), metal chelating compounds such as siderophores and biosurfactants [95,96].
helps plants to grow well even under metal strain condition. Bacteria, such as Bacillus mucilaginosus (K-soluble), Bacillus megaterion (P-soluble) and Azotobacter chroococcum (Nitrogen-fixing) can help plant growth in several ways i.e. lowering pH, producing plant growth regulations such as Indoleacetic Acid (IAA), metal chelating compounds such as siderophores and biosurfactants [95,96].
Pb accumulation is increased by upto 131% with help of Pseudomonas fluoresce and 80% with microbacterium species in Brassica napus shoots. In addition to secreting extractable metals and organic substrate for facilitating plant growth and develfigopment by microorganism, plants also produce certain enzymes to degrade organic contaminants in soils.
Limitation of Phytoremediations
Although phytoremediation is non-destructive, solar driven techniques for heavy metals accumulation and removal from soil, it also has some limitations [78,97].
1. Time consuming (several years) for cleanup.
2. Slow growth and development of many hyperaccumulators.
3. Biotic factors and disease attack may be compromised accumulation capacity of hyperaccumulatiors.
4. Climate and weather conditions affect hyperaccumulator plants performance.
5. Only effective in low and moderated level of contamination.
6. Limited bioavailability, hard to be mobilized more tightly bound fraction of metal ions from soil.
7. Risk of food web contamination in case of mismanagement.
Future Prospects
Phytoremediation utilize unique properties of hyperaccumulator of plants to act as pumping machine for HM removal from soil and water. Presently, phytoremediation is at its infant stage which requires novel strategies for its development. This can be achieved either by exploring vast diversity of hyperacummulators or through gene manipulation by genetic engineering.
Introduction of foreign tolerant genes in plants to cleanup heavy metal contamination from soil and water is feasible. Although several research groups have established ideal hyperaccumulaor to accumulate, translocate and detoxify HM through genetic engineering. However, no ideal plant can be established for hyperaccumulator and hypertolerance until the availability of complete genome information is ensured.
Transgene hyperaccumulator plant microbe interaction is highly efficient in absorbing, accumulate and translocation of HMs in plants. Therefore finding and establishing appropriate microorganisms (bacteria, fungi) for phytoremediation requires adequate attention. Proper agronomic management practices of using chelate assisted remediation in combination of transgenic traditional approach need to be explored for accumulation of HM.
There is an urgent need to understand the role of plant hormone (IAA, GA cytokine) to increase potential of hyperaccumulator plants. In combination of soil microbe and plants, using different technologies can be promising way for sustainable remediation and environment safety. Interdisciplinary research of plant biochemist, physiologist, soil microbiologist, ecologist and soil chemists would help to answer many limitation/challenges faced in phytoremediation. Researchers need to identify and recommend commercial application of phytoremediation to remove contaminations from soil and water.
Conclusion
Since soil contamination with heavy metals is a serious worldwide concern, therefore eco-friendly and solar driven technology which has community acceptance need to be explored. Phytoremediation is one of such approach which needs to be explored further for the removal of contamination. This review reveals that cost effective phytoremediation technology can be used to remediate HMs and pollutants from contaminated soil and water. However, it requires better understanding about different steps/processes involved in removal by hyperaccumulators. A concise overview of numerous approaches of phytoremediation in this review demonstrates that despite some limitations, this has numerous advantages and can be applied to remediate toxins from soil and water.
References
- Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C. A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem. 2016.
- Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Sabir M. Heavy metal stress and crop productivity. Hakeem KR, editor. In: Crop Production and Global Environmental Issues. Springer International Publishing. 2015; 1-25.
- Hediji H, Djebali W, Belkadhi A, Cabasson C, Moing A, Rolin D, et al. Impact of long-term cadmium exposure on mineral content of Solanum lycopersicum plants: Consequences on fruit production. S Afr J Bot. 2015; 97: 176–181.
- Sabir M, Waraich EA, Hakeem KR, Ozturk M, Ahmad HR, Shahid M. Phytoremediation. Hakeem K, Sabir M, Ozturk M, Murmet A, editors. In: Soil Remediation and Plants: Prospects and Challenges, contaminated soil is indispensable. Elsevier, Boston. 2015; 85-105.
- Baker A, Brooks R, Reeves R. Growing for gold, copper and zinc. New Scient. 1988; 10: 44-48.
- Bhargava A, Carmona FC, Bhargava M, Srivastava S. Review: Approaches for enhanced phytoextraction of heavy metals. J Environ Manage. 2012; 105: 103-120.
- Sancenon V, Puig S, Mira H, Thiele DJ, Penarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol. 2003; 51: 577-587.
- Glass D. U.S. and International Markets for Phytoremediation. In: Reports D. Glass Associates Inc, Needham, MA, USA 1999–2000.
- Tsao DT. Overview of phytotechnologies. Advan Biochem Engi Biotech. 2003; 78: 1-50.
- Raskin I, Ensley B. Phytoremediation of toxic metals: using plants to clean up the environment. Wiley-Interscience New York. 1999.
- EEA: European Environment Agency: Progress in Management of Contaminated Sites (CSI 015). 2007.
- Ali H, Khan E, Sajad MA. Phytoremediation of heavymetals concepts and applications. Chemosphere. 2013; 91: 869–881.
- Alloway BJ. Heavy metals in soils. 2nd Edn. Glasgow, UK: Blackie Edn. The University of Reading. 1995.
- Kramer U, Chardonnens AN. The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbiol Biotech. 2001; 55: 661-72.
- Gupta M, Cuypers A, Vangronsveld J, Clijsters H. Copper affects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol Plant. 1999; 106: 262-267.
- Schutzendubel A, Polle A. Plant responses to abiotic stresses: heavy metalinduced oxidative stress and protection by mycorrhization. J Experi Bot. 2002; 53: 1351-65.
- Murphy A, Eisinger W, Shaff J, Kochian L, Taiz L. Early copper-induced leakage of K+ from arabidopsis seedlings is mediated by ion channels and coupled to citrate efflux. Plant Physiol. 1999; 121: 1375-1382.
- Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochemicaet Biophysica Acta-Mol Cell Res. 2006; 1763: 595-608.
- Hansch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opi Plant Biol. 2009; 12: 259-266.
- Pilon M, Cohu CM, Ravet K, Abdel-Ghany SE, Gaymard F. Essential transition metal homeostasis in plants. Curr Opi Plant Biol. 2009; 12: 347-357.
- Yadav SK. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afri J Bot. 2009; 76: 167-179.
- Pilon-Smits E. Phytoremediation. Ann Rev Plant Biol. 2005; 56: 15-39.
- Punshon T, Dickinson NM. Acclimation of Salix to metal stress. New Phytolo. 1997; 137: 303-314.
- Dutton MV, Humpreys P. Assessing the potential of short rotation coppice (SRC) for cleanup of radionuclide- contaminated sites. Inter J Phytopathol. 2005; 7: 279-293.
- Aronsson P, Perttu K. Willow vegetation filters for wastewater treatment and soil remediation combined with biomass production. Forestry Chron. 2001; 77: 293-299.
- Di Baccio D, Tognetti R, Sebastiani L, Vitagliano C. Responses of Populus deltoids x Populusnigra (Populus x euramericana) clone I-214 to high zinc concentrarions. New Phytol. 2003; 159: 443-452.
- Sebastiani L, Scebba F, Tognetti R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoids x maximowiczii) and I-214 (P. x euramericana) exposed to industrial waste. Environ Experi Bot. 2004; 52: 79-88.
- Soudek P, Tykva R, Vanek T. Laboratory analysis of Cs-137 uptake by sunflower, reed and poplar. Chemosphere. 2004; 55: 1081-1087.
- Dos Santos U, Wieshammer MN, Vega GR, Wenzel WW. Hydroponic screening for metal resistence and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut. 2007; 148: 155-165.
- Kopponen P, Utriainen M, Lukkari K, Suntionen S, Karenlampi L, Karenlampi S. Clonal differences in copper and zinc tolerance of birch in metalsupplemented soils. Environ Pollut. 2001; 112: 89-97.
- Laureysens I, Blust R, Temmerman L, Lemmens C, Ceulemans R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture: Seasonal variation in leaf, wood and bark concentrations. Environ Pollut. 2004; 131: 485-494.
- ZalesnyJr, Bauer RS, Hall EO, Zalesny RB, Kunzman JA, Rog J, et al. Clonal variation in survival and growth of hybrid poplar and willow in an in situ trial on soils heavily contaminated with petroleum hydrocarbons. Inter J Phytoreme. 2005; 7: 177-197.
- Raskin I, Robert DS, David ES. Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opi Biotech. 1997; 8: 221-226.
- Michael JH, Christopher SC. Transporters of ligands for essential metal ions in plants. New Phytolo. 2007; 174: 499-506.
- Guerra L, Guidi R, Slot I, Callegri S, Sompallae R, Pickett CL, et al. Bacterial genotoxin triggers FEN1-dependent RhoA activation, cytoskeleton remodeling and cell survival. J Cell Sci. 2011; 124: 2735-42.
- Delhaize E, Ryan PR, Randall PJ. Aluminium tolerance in wheat (Triticum aestivum L.) (II. Aluminium-stimulated excretion of malic acid from root apices). P Physiol. 1993; 103: 695-702.
- Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorus efficiency. Ann Rev Plant Biol. 2004; 55:459-493.
- Qin R, Hirano Y, Brunner I. Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physiol. 2007; 27: 313-320.
- Takagi SI, Nomoto K, Takemoto T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutri. 1984; 7: 1-5.
- Zhao Y, Chengcai C. Towards Understanding Plant Response to Heavy Metal StressProf. Arun Shanker, editor. In: Abiotic Stress in Plants-Mechanisms and Adaptations, ISBN: 978-953-307-394-1, In Tech, DOI: 10.5772/24204. 2011.
- Baker AJM, McGrath SP, Reeves RD, Smith JAC, Terry N, Banuelos G, Vangronsveld J, editors. Metal hyperaccumulator plants: a review of the ecology and physiology of a biochemical resource for phytoremediation of metal-polluted soils. In: Phytoremediation of Contaminated Soil and Water. Lewis Publishers. Boca Raton, FL, USA. 2000; 85-107.
- Smith SE, Reed DJ. Mycorrhizal Symbiosis, second ed. Academic Press, London. 1997.
- Galli U, Schuepp H, Brunold C. Heavy metal binding by mycorrhizal fungi. Physiol Plant. 1994; 92: 364-368.
- Chaudhry, TM, Hill L, Khan AG, Kuek C, Wong MH, Wong JWC, Baker AJM, editors. Colonization of iron and zinc-contaminated dumped ®ltercake waste by microbes, plants and associated mycorrhizae. In: Remediation and Management of Degraded Land, CRC Press LLC, Boca Raton. 1999; 275- 283.
- Raman N, Nagarajan N, Gopinathan S, Sambandan K. Mycorrhizal status of plant species colonizing a magnesite mine spoil in India. Biol Ferti Soils. 1993; 16: 76-78.
- Raman N, Sambandan S. Distribution of VAM fungi in tannery e.uent polluted soils of Tamil Nadu, India. Bull. Environ Contamin Toxicol. 1998; 60: 142-150.
- Weissenhorn I, Leyval C. Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomusmosseae and cadmium uptake in sand culture. Plant Soil. 1995; 175: 233-238.
- Pawlowska TE, Blaszkowski J, Ruhling A. Themycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza. 1996; 6: 499-505.
- Turnau K. Heavy metal content and localization in mycorrhizal Euphorbia cyparissias from zinc wastes in Southern Poland. Acta Soc Bot Pol. 1998; 67: 105-113.
- Joner EJ, Leyval C. Uptake of Cd by roots and hypae of a Glomusmosseae/ Trifolium subterraneum mycorhiza from soil amended with high and low concentration of cadmium. New Phytol. 1997; 135: 353-360.
- Killham K, Firestone MK. Vesicular arbuscularmycorrhizal mediation of grass response to acid and heavy metal deposition. Plant Soil. 1986; 72: 39-48.
- Wang J, Evangelou V, M Pessarakli, Marcel Dekker. Metal tolerance aspects of plant cell wall and vacuole. In: ed, Handbook of plant and crop physiology, Inc, New York. 1995; 695-717.
- Konno H, Nakato T, Nakashima S, Katoh K. Lygodium japanicum fern accumulates copper in the cell wall pectin. J Experi Bot. 2005; 56: 1923-1931.
- Bringezu K, Lichtenberger O, Leopold I, Neumann D. Heavy metal tolerance of Silene vulgaris. J Plant Physiol. 1999; 154: 536-546.
- Palmer CM, Guerinot ML. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nature Chem Biol. 2009; 5: 333-340.
- Williams L, Mills R. P1B-ATPases – an ancient family of transition metal pumps with diverse functions in plants. Tren Plant Sci. 2005; 10: 491-502.
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA. 1998; 95: 7220-7224.
- Guerinot ML, Eidet D. Zeroing in on zinc uptake in yeasts and plants. Curr Opi Plant Biol. 1999; 2: 244-249.
- Gaxiola RA, Fink GR, Hirski KD. Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiol. 2002; 129: 967-973.
- Obata H, Umebayashi M. Production of SH compounds in higher plants of different tolerance to Cd. Plant Soil. 1993; 155: 533-536.
- Pietrini F, Zacchini M, Iori V, Pietrosanti L, Bianconi D, Massacci A. Screening of poplar clones for cadmium phytoremediation using photosynthesis, biomass and cadmium content analysis. Inter J Phytoreme. 2010; 12: 105- 120.
- Ahner BA, Morel FMM. Phytochelatin production in marine algae: Induction by various metals. Limnol Oceanogr. 1995; 40: 658–665.
- Kupper, H, Peter MHK. Heavy metal uptake by plants and cyanobacteria. Metal Ions Biolo Syst. 2005; 44: 97-101.
- Cobbett CS. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol. 2000; 3: 211-216.
- Cobbett C, Goldsbrough P. Phytochelatins and metalliothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002; 53: 159-182.
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. MTP1- dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 2009; 57: 1116-1127.
- Arrivault S, Senger T, Kramer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006; 46: 861-879.
- Desbrosses-Fonrouge AG, Voigt K, Schroder A, Arrivault S, Thomine S, Kramer U. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS letters. 2005; 579: 4165-4174.
- Gravot A, Lieutaud A, Verret F, Auroy P, Vavasseur A, Richaud P. AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS letters. 2004; 561: 22-28.
- Puig S, Penarrubia L. Placing metal micronutrients in context: transport and distribution in plants. Curr Opi Plant Biol. 2009; 12: 299-306.
- Shikanai T, Muller MP, Munekage Y, Niyogi KK, Pilon M. PAA1, a P-Type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell. 2003; 15: 1333-1346.
- Andres-Colas N, Sancenon V, Rodriguez-Navarro S, Mayo S, Thiele D, Ecker J, et al. The Arabidopsis heavy metal P-type ATPases HMA5 interacts with metallochaperons and functions in copper detoxification of roots. Plant J. 2006; 45: 225-236.
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008; 453: 391-395.
- Wong CKE, Cobbett CS. HMA type ATPAses are the major mechanism for root to shoot translocation in Arabidopsis thaliana. New Phytolo. 2009; 181: 71–78.
- Kobayashi T, Nakanishi H, Takahashi M, Mori S, Nishizawa N. Generation and field trials of transgenic rice tolerant to iron deficiency. Rice. 2008; 1: 144-153.
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signalling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005; 17: 1866-1875.
- Meena D, Singh H, Chaudhari SK. Elucidating strontium response on growth dynamics and biochemical change in Phaseolus mungo L. Inter J Agricul Environ Biotech. 2011; 4: 107-113.
- Kumar PBAN, Dushenkov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995; 29: 1232–1238.
- Mahar A, Wang P, Ali A, Awasthi Mk, Lahori Ah, Wang Q, Et Al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol Environ Saf. 2016; 126: 111–121.
- Pollard AJ, Powell KD, Harper FA, Andrew J, Smith C. The genetic basis of metal hyperaccumulation in plants. Criti Rev Plant Sci. 2002; 21: 539-566.
- Bolan N, Kunhikrishnan A, Thangarajana R, Kumpiene J, Park J, Makino T, et al. Remediation of heavy metal (loid)s contaminated soils to mobilizeor to immobilize. J Hazard. Mater 2014; 266: 141-166.
- McGrath SPFJ, Zhao, Lombi E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil. 2001; 232: 207- 214.
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol. 1994; 127: 61-68.
- Guo J, Xu W, Ma M. The assembly of metals chelation by thiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J. Hazard. Mater. 2012; 200: 309-313.
- Dushenkov VL, Kumar PBAN, Motto H, Raskin I. Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol. 1995; 29: 1239-1245.
- Augustynowicz J, Tokarz K, Baran A, Plachno BJ. Phytoremediation of water polluted by thallium, cadmium, zinc, and lead with the use of macrophyte Callitriche cophocarpa. Arch Environ Contam Toxicol. 2014; 66: 572-581.
- Dimitroula H, Syranidou E, Manousaki E, Nikolaidis NP, Karatza, GP, Kalogerakis N. Mitigation measures for chromium-VI contaminated groundwater-the role of endophytic bacteria in rhizo?ltration. J. Hazard. Mater. 2015; 281: 114-120.
- Ignatius A, Arunbabu V, Neethu J, Ramasamy EV. Rhizofiltration of lead using an aromatic medicinal plant Plectranthus amboinicus cultured in a hydroponic Nutrient Film Technique (NFT) system. Environ Sci Pollut Res. 2014; 21: 13007-13016.
- Jasrotia S, Kansal A, Mehra A. Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl Water Sci. 2015; 2: 889-896.
- Laureysens I, De Temmerman L, Hastir T, Van Gysel M, Ceulemans R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture: Vertical distribution and phytoextraction potential. Environ Pollut. 2005; 133: 541-551.
- Baudouin C, Charveron M, Tarrouse R, Gall Y. Environmental pollutants and skin cancer. Cell Biol Toxicol. 2002; 18: 341-348.
- Knasmuller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, God R, et al. Detection of genotoxic effects of heavy metal contaminated soils with plant bioassays. Mutation Res. 1998; 420: 37-48.
- Hooda V. Phytoremediation of toxic metals from soil and waste water. J Environ Biol. 2007; 28: 367-376.
- Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol. 2000; 3: 153-62.
- Ullah R, Ahmad S, Atiq A, Hussain H, Rehman N, Naser M, et al. Quatification and antibacterial activity of flavonoids in coffee samples. Afr J Tradit Complement Altern Med. 2015; 12: 84-86.
- Ahmed E, Holmstrom SJM. Siderophores in environmental research: roles and application. Microb Biotechnol. 2014; 7: 196-208.
- Ramamurthy AS, Memarian R. Phytoremediation of mixed soil contaminants. Water Air Soil Pollut. 2012; 223: 511-518.