
Short Communication
Austin Biochem. 2017; 2(2): 1013.
G-Protein Linked Effect of Pasteurella Multocida Toxin on GABA Outflow from Neocortical Synaptosomes
Moser A¹*, Orth JH² and Feuerstein TJ³
¹Department of Neurologyc and Freiburg Institute for Advanced Studies (FRIAS), University of Luebeck and University of Freiburg, Germany
²Institute for Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Germany
³Section of Neuroelectronic Systems of the Department of Neurosurgery, University of Freiburg, Germany
*Corresponding author: Moser A, Neurochemical Research Group, Department of Neurology and Freiburg Institute for Advanced Studies (FRIAS), University of Luebeck and University of Freiburg, Ratzeburger Allee 160, D-23538 Luebeck, Germany
Received: October 10, 2017; Accepted: November 29, 2017; Published: December 06, 2017
Abstract
Pasteurella Multocida Toxin (PMT) stimulates diverse cellular signal transduction pathways by activating heterotrimeric G proteins through deamidation of a a-subunit glutamine residue of the G-protein. Since mammalian cells are able to take up PMT probably by receptor-mediated endocytosis, the question was raised whether PMT is capable of modulating γ-aminobutyric acid (GABA) outflow from neocortical synaptosomes of the rat in vitro. In our experiments PMT did not modify basal GABA outflow. However, GABA release induced by potassium ions was significantly reduced. Here the toxin effect was not modulated by the GABAB-receptor agonist baclofen. These results let us to suggest that PMT activates G-proteins of the inhibitory metabotropic GABAB autoreceptor on GABAergic nerve terminals.
Keywords: G-protein; Pasteurella multocida toxin; γ-aminobutyric acid; Synaptosomes; Rat
Abbreviations
PMT: Pasteurella Multocida Toxin; GABA: γ-Aminobutyric Acid; GTP: Guanosine Triphosphate; AUC: Area Under the Curve; GABA-R: GABA Receptor; Bcl: Baclofen
Introduction
The Gram negative opportunistic bacterium Pasteurella multocida produces a 146-kDa protein toxin (Pasteurella multocida toxin, PMT) which stimulates diverse cellular signal transduction pathways by activating heterotrimeric G proteins, thereby arresting the G protein in the active state [1]. The toxin substrates primarily are Gaq, Ga12/13 and the Gai-family proteins. The toxin deamidates a glutamine of the a-subunits of heterotrimeric G-proteins [2]. This glutamine is essential for hydrolyzing GTP. The question was raised whether PMT is intracellularly capable of modulating neurotransmitter release from neocortical synaptosomes of the rat in vitro, since cellular (or synaptosomal) up-take of PMT by mammalian cells was described through receptor-mediated endocytosis [3].
Materials and Methods
Animal experiments were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and all efforts were made to minimize both suffering and number of animals. Wistar rats (weighing 200-300 g, N=6, University of Freiburg, Germany) were decapitated under CO2 anesthesia, and the brains were carefully removed. The neocortex was dissected and immediately placed in ice-cold saline.
The buffer used for the preparation, suspension, incubation, and superfusion of synaptosomes contained (mM): NaCl 121, KCl 1.8, CaCl2 1.3, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 10, and was saturated before use with 95% O2/5% CO2; pH 7.4. Synaptosomes were preincubated during 30 min, 37°C, 95% O2/5% CO2 with Pasteurella multocida toxin (PMT, 100 nM). To study the release of endogenous GABA, synaptosomes were then transferred to glass microfiber GF/B filters (Whatman, Dassel, Germany) into 24 superfusion chambers within a water bath (37°C). Synaptosomes were covered with GF/B filters within the superfusion chambers to prevent their wash out.
Then, collection of four fractional samples followed (5 min intervals, 0.2 ml/min flow rate; 37°C, presence of baclofen as indicated). A 10 min interval of superfusion before stimulation corresponded to resting conditions. Synaptosomes were stimulated for 2 min using KCl 15 mM.
The GABA contents of fractional samples and of synaptosomes were determined by High Performance Liquid Chromatography (HPLC). After pre-column derivatization with o-phthaldialdehyde and sodium sulfite for 30 min, GABA values were measured using HPLC with electrochemical detection. The HPLC system consisted of a C18 column (Eurospher 100, 5 μm, column size 250x4 mm) and a pre-column (30x4 mm). The isocratic mobile phase (0.1 M sodium phosphate buffer, pH 4.5, containing 0.5 mM EDTA and 25% methanol) was previously degassed using helium and pumped at a flow rate of 1.0 ml/min. The compounds were detected electrochemically using a glassy carbon electrode set at a potential of 900 mV, relative to an Ag/AgCl reference electrode (Waters 460 electrochemical detector, Millipore Corporation, Eschborn/Ts., Germany).
GABA outflow was expressed in nM, as mean ± Standard Error of the Mean (SEM). The average of baseline samples was defined as 100%. Differences between the means of treatments and their corresponding controls were tested with one-way Analysis of Variance (ANOVA). Subsequent multiple comparisons were made by the Kruskal-Wallis test and t-test for pair wise comparisons as indicated. Areas Under the Curve (AUC) were calculated by GraphPad Prism software. Statistical significance was set at p< 0.05.
Results and Discussion
Synaptosomes are isolated nerve terminals and serve as an important model system for studying the molecular mechanisms of synaptic function in the brain [4]. In our preparation of rat neocortical synaptosomes, GABA outflow was 15.0 ± 3 nM under basal conditions. Preincubation of synaptosomes with Pasteurella multocida toxin (PMT, 100 nM) did not modify basal GABA outflow (14.2 ± 2 nM). The GABA amounts per fraction were comparable to those found by Rassner and co-workers [5] in synaptosomes of rat and also human neocortex. Here fluorescence-activated particle sorting experiments corroborated GABAergic synaptosomes to be highly present in such preparations [5,6].
Potassium ions (15 mM) significantly increased outflow of vesicular GABA to 28.8 ± 3.7 nM, as described earlier [7,8]. This potassium-induced enhancement was completely abolished under PMT conditions (Figure 1), most probably through G-protein activation. GABAB Receptors (GABAB-R) are metabotropic, composed of 7 transmembrane domain subunits linked to G-proteins [9], and also presynaptically located on GABAergic synaptic terminals as autoreceptors [10,11]. As all GABAB-R subtypes these autoreceptors are inhibitory receptors functionally [8,10]. During Baclofen (Bcl) incubation, i.e. by activation of GABAB-R, GABA outflow significantly decreased and no further potassium-induced stimulation could be observed (Figure 1). In the case of PMT preincubation, the inhibitory baclofen effect (0.5 or 5 mM, respectively) remained unchanged, without any further decrease of GABA outflow (Figure 1). The finding that the effects of PMT and baclofen are not additive implicates activation of the same effector cascade. The receptor agonist baclofen activates GABAB-R from the extracellular site while PMT stimulates the G-protein complex of the GABAB-R unit intracellularly as shown in (Figure 2). For this, it is necessary for PMT to pass through cell or as in our experiments the synaptosomal membrane by receptormediated endocytosis as proposed by Pettit and co-workers [3]. Since PMT uptake could be competed with mixed gangliosides, the receptor was suggested to belong to this class [3]. Although the precise mechanism of translocation of PMT into the cytosol remains to be elucidated [2], the effects of PMT can, additionally, be used as a tool for biochemical studies like other bacterial toxins as pertussis or cholera toxin that also modulate G-proteins [12,13].
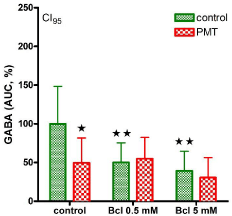
Figure 1: GABA outflow in the presence of potassium ions (15 mM) was
given as area under the curve (%). In the verum group pasteurella multocida
toxin (PMT, 100 nM) was preincubated for 30 min. Baclofen (Bcl) 0.5 or 5 mM
was added as indicated. *significant, when compared to control; **significant,
when compared to –Bcl; p< 0.05.
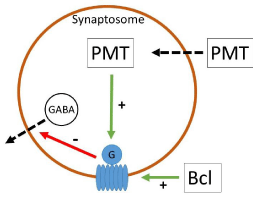
Figure 2: The receptor agonist baclofen activates the GABAB-R binding
site while PMT stimulates the G-protein complex of the GABAB-R unit from
intracellular. Both are inhibiting vesicular GABA outflow.
Conclusion
The results described above give evidence that Pasteurella multocida toxin may pass through cellular membranes into synaptosomes where it activates G-proteins associated to the GABAB autoreceptor inhibiting release of vesicular GABA from neocortical synaptosomes of the rat.
References
- Orth JH, Preuss I, Fester I, Schlosser A, Wilson BA, Aktories K. Pasteurellamultocida toxin activation of heterotrimeric G proteins by deamidation. Proc Natl Acad Sci. 2009; 106: 7179-7184.
- Orth JH, Fester I, Siegert P, Weise M, Lanner U, Kamitani S, et al. Substrate specificity of Pasteurella multocida toxin for a subunits of heterotrimeric Gproteins. FASEB J. 2013; 27: 832-842.
- Pettit RK, Ackermann MR, Rimler RB. Receptor-mediated binding of Pasteurella multocida dermonecrotic toxin to canine osteosarcoma and monkey kidney (vero) cells. Lab Invest. 1993; 69: 94-100.
- Evans GJ. The synaptosome as a model system for studying synaptic physiology. Cold Spring Harb Protoc. 2015; 421-424.
- Rassner MP, Moser A, Follo M, Joseph K, van Velthoven-Wurster V, Feuerstein TJ. Neocortical GABA release at high intracellular sodium and low extracellular calcium: an anti-seizure mechanism. J Neurochem. 2016; 137: 177-189.
- Biesemann C, Grønborg M, Luquet E, Wichert SP, Bernard V, Bungers SR et al. Proteomic screening of glutamatergic mouse brain synaptosomes isolated by fluorescence activated sorting. EMBO J. 2014; 33: 157-170.
- Mantovani M, Van Velthoven V, Fuellgraf H, Feuerstein TJ, Moser A. Neuronal electrical high frequency stimulation enhances GABA outflow from human neocortical slices. Neurochem Int. 2006; 49: 347-350.
- Mantovani M, Moser A, Haas CA, Zentner J, Feuerstein TJ. GABAA autoreceptors enhance GABA release from human neocortex: towards a mechanism for High Frequency Stimulation (HFS) in brain?. Naunyn- Schmiedberg´s Arch Pharmacol. 2009; 380: 45-58.
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH et al. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998; 396: 679-682.
- Kobayashi M, Takei H, Yamamoto K, Hatanaka H, Koshikawa N. Kinetics of GABABautoreceptor-mediated suppression of GABA release in rat insular cortex. J Neurophysiol. 2012; 107: 1431-1442.
- Fassio A, Bonanno G, Cavazzani P, Raiteri M. Characterization of the GABA autoreceptor in human neocortex as a pharmacological subtype of the GABAB receptor. Eur J Pharmacol. 1994; 263: 311-314.
- Moser A, Cramer H. Peptidergic modulation of G-protein coupled cyclic AMP accumulation in the ratcaudate nucleus. Neuropeptides. 1992; 22: 143-147.
- Moser A, Klose C. Somatostatin modulation of adenosine coupled G-protein subunits in the caudatenucleus of the rat. Neuropeptides. 1993; 34: 293-297.