
Original Article
Austin Biochem. 2017; 2(2): 1014.
Determination of Piperidine Alkaloids from Indian Tobacco (Lobelia inflata) Plants and Plant-Derived Products
St-Pierre A¹, Lajeunesse A1,2 and Desgagné-Penix I1,2*
¹Department of Chemistry, Biochemistry and Physics, Université du Québec à Trois-Rivières, Canada
²Forensic Research Group, Université du Québec à Trois- Rivières, Canada
*Corresponding author: Desgagné-Penix I, Forensic Research Group, Université du Québec à Trois-Rivières, 3351 boul. des Forges, Trois-Rivières, QC, G9A 5H7, Canada
Received: November 15, 2017; Accepted: December 06, 2017; Published: December 13, 2017
Abstract
Lobelia inflata also known as Indian tobacco is a traditional medicinal plant native to North America. L. inflata contains piperidine alkaloids that constitute a large family of specialized metabolites, many of which are of great interest for their various pharmaceutical activities. To date, no studies have reported on the alkaloid profile of various L. inflata products. This study aims to characterize the chemical composition of L. inflata products using several methods. Efficient Thin-Layer Chromatography (TLC), High-Performance Liquid-Chromatography (HPLC or LC) and LC-tandem Mass Spectrometry (LC-MS/MS) methods were developed for the analysis of piperidine alkaloids from extracts of L. inflata whole plants and from Lobelia-derived natural products including tincture, capsule and tobacco in order to determine the alkaloid profiles of these samples. Several piperidine alkaloids including 8,10-diethyl-lobelionol, norlelobanidine and lobeline were detected and identified from the sample extracts. Tincture did not contain lobeline. Therefore, the Lobelia-derived products have to be selected with caution if intended for pharmaceutical use.
Keywords: Lobelia inflata; Campanulaceae; Indian tobacco; Piperidine alkaloids; High-Performance Liquid Chromatography (HPLC); Liquid Chromatography tandem Mass Spectrometry (LC-MS/MS); Lobeline
Introduction
Lobelia inflata L. (Indian tobacco, Pukeweed) is a traditional medicinal plant of the Campanulaceae family native to eastern North America including southeast Canada and eastern United States [1,2]. It has long been used by aboriginal Amerindians for medicinal and religious purposes. Nowadays, Indian tobacco and its derived products are used to treat asthma and bronchitis, to repel bugs, and to relieve respiratory and muscular disorders. Lobelia plants produce bioactive piperidine alkaloids, including lobeline, which is used in the treatment of addictions [3,4]. Experimental and clinical research studies on Lobelia piperidine alkaloids have determined that they have many pharmacological properties including stimulant, diuretic, expectorant, antimicrobial and anti-cancer [3-6]. Also it was reported that piperidine alkaloids act on the nicotinic acetylcholine receptors and interact with neurotransmitter transporters such as transporters for dopamine, serotonin and monoamine vesicle transporter [6]. This type of interaction in the central nervous system makes this plant interesting not only for its beneficial effect in the treatment of addiction but also for the treatment of diseases such as Alzheimer’s and Parkinson’s diseases [6,7].
Unlike the abundant literature on the pharmaceutical effects of piperidine alkaloids, information on the biochemistry and metabolic pathways is incomplete. Previous studies using radio-labeled precursors have led to biochemical elucidation of the first reactions [8-10]. However, no gene involved in biosynthesis has been identified or characterized to date. The piperidine alkaloid biosynthesis begins with the formation of the piperidine ring derived from lysine. The first reaction is catalyzed by a Lysine Decarboxylase (LDC) to yield cadaverine (Figure 1). From there, a series of reactions likely involving a deamination, oxidation and imine formation yields the piperidinium cation (Figure 1). Benzoylacetic acid is another proposed precursor necessary for Lobelia alkaloid biosynthesis. It is most likely derived from phenylalanine through the phenylpropanoid pathway. The first reaction involves Phenylalanine Ammonia-Lyase (PAL) to yield cinnamic acid, then hydroxylation and oxidation are required to form the benzoylacetic acid (Figure 1). Lobelia alkaloids present several substitutions at the C2 and C6 positions of the piperidine ring. These substituents vary from a phenyl ring to acetate side chain. Thus, we divided Lobelia alkaloids into three groups. Group I comprises substitutions like formate or acetate side chains including 8-methyl- 10-ethyl-lobelionol and 8,10-diethyl-lobelionol. Group II includes one phenyl substitution such as allosedamine and norlelobanidine whereas group III comprises two phenyl substitutions such as lobeline (Figure 1). It should be noted that the exact sequence of reactions leading to these conversions is not yet known. Figure 1 shows the hypothetical biosynthesis suggesting that L-lysine is the precursor of the central piperidine ring while the side chains are derived from other pathways.
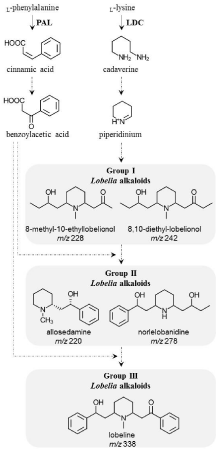
Figure 1: Proposed biosynthetic pathway leading to Lobelia piperidine
alkaloids. Biosynthetic enzymes Phenylalanine Ammonia-Lyase (PAL) and
Lysine Decarboxylase (LDC) are proposed to participate in the production of
precursors, benzoylacetic acid and piperidium, for the synthesis of piperidine
alkaloids. Dotted arrows indicate more than one catalytic conversion. Lobelia
alkaloids were divided in three groups: group I includes alkaloids with
piperidine side chains without phenyl groups, group II comprises alkaloids
with one phenyl group on one of the side chain and group III with two phenyl
groups. Examples for each group are represented in the figure with their
corresponding mass-to-charge (m/z) [M+H].
Owing to the pharmaceutical potential of Lobelia alkaloids, interests toward its metabolism and phytochemical content are rising. However, due to difficulties and high cost of industrial synthesis, Lobelia alkaloids continue to be extracted from plants. Given the increasing demand for herbal medicine, new resources for these specialized metabolites need to be investigated. Lobelia-derived products are available in supplements such as tinctures and as dried herb in capsules. However, the content of piperidine alkaloids in these forms is not known. In addition, few studies have reported on the alkaloid profiles of L. inflata plants. The aim of this study was to determine the chemical composition of L. inflata plant and Lobeliaderived products specifically targeting the piperine alkaloids. This characterization will allow for a better comprehension of alkaloid profile in plant and plant-derived products specifically on the relative abundance, stability and potential degradation metabolite of piperine alkaloids.
Experimental Procedure
Chemicals, reagents and materials
Organic solvents such as methanol, chloroform, acetonitrile, ethanol, for chromatography (HPLC grade) and Mass Spectrometry (LC-MS grade) were purchased from Fisher Scientific (https://www. fishersci.ca). Milli-Q water was prepared with a water purification system of Merck Millipore (MA, USA). Standards such as piperine and lobeline were obtained from Sigma-Aldrich (ON, Canada).
Plant materials
Plants of Lobelia inflata were kindly provided by the Montréal Botanical Garden (Québec, Canada) and were used to extract alkaloids. In addition, three different commercial products, claimed to be made from Lobelia, were used including a liquid tincture (Nature’s Answer Homeopathic tincture of L. inflata), dried capsules (Nature’s Way Lobelia supplement) and a bag of Indian tobacco (Feel Good Natural dried Lobelia herbs) (Supplementary data 3).
Metabolite extraction
Whole fresh L. inflata plant samples (leaves, roots and stem) were ground to fine powder, using liquid nitrogen and a pestle and mortar. Commercial samples (liquid tincture, capsule and dried Indian tobacco) were used as such. Two grams (or 2 mL tincture) of each sample, in triplicates, were measured in glass tubes and 250 ppm piperine (Internal Standard (IS); Sigma-Aldrich+) was added. Samples were extracted with 20 mL of 100% methanol (HPLC grade; Fisher Scientific) for 24 hours at room temperature. Samples were centrifuged at 12000 rpm for 10 minutes to remove debris and the supernatants were transferred to new tube to evaporate. To speed up and facilitate evaporation, this step was carried out under a nitrogen gas flow in a heating block at 50°C. A liquid-liquid acidbase extraction was performed to extract alkaloids. As such, dried samples were solubilized with 450 μL of 2 % H2SO4 (Fisher Scientific), vortexed and extracted with 450 μL of chloroform (Fisher Scientific) to remove the neutral undesirable compounds. The acidic aqueous phase was separated from the organic phase and basified with 150 μL of NH3 (7N; Fisher Scientific). The aqueous phase was washed 2X with 450 μL chloroform. The organic layer containing alkaloids was collected, evaporated to dryness at room temperature and solubilized with 300 μL methanol. Each sample were extracted in triplicate and aliquotes were stored in freezer until analysis.
Thin Layer Chromatography (TLC) analysis
Initial visualization of alkaloids using TLC allowed for a quick and qualitative analysis of compound present in the samples. A total of 10 μL for each sample or lobeline (1 mg/mL; Sigma-Aldrich) standard were spotted on a silica gel 60 F254 TLC plate (EMD Millipore). The loaded plate was placed in a pre-equilibrated migration chamber with mobile phase chloroform:methanol:ammonia (90 : 9 : 1) and allowed to separate. After migration was complete, the plate was completely dried and visualized with a UV illumination at wavelengths of 254 nm and 365 nm. Also, visualization of alkaloids was performed using Dragendorff’s reagent [11]. The alkaloids were compared with each other and with the standard according to their migration distance relative to the solvent front (Rf).
High Performance Liquid Chromatography HPLC-DAD conditions
To achieve a fast scanning and to know relative retention time of alkaloids present in Lobelia extracts, a HPLC Prominence-i LC- 2030 LT consisting of a quaternary gradient pump with an integrated degaser, a Photodiode Array (PDA) detector, and an autosampler was used (Shimadzu, MD, USA). Compounds were separated onto a Kinetex EVO C18 reverse phase column (5μm, 150 mm×4.6 mm, 100 Å) (Phenomenex, CA, USA) integrated with a security Guard ULTRA cartridfes EVO-C18 (4.6 mm internal diameter) pre-column (Phenomenex, CA, USA). The column temperature was set at 40°C and the injection volume was established at 10 μL. Gradient elution was applied with 20 mM ammonium formate, pH 3 (solvent A) and acetonitrile 100% (solvent B). The separation was performed at a constant flow rate of 0.5 mL/min with the following conditions : a linear gradient from 10% to 30% of B in 5 min; 5-20 min 30% B; 20-22 min 90% B; 22-24 min 90% B; 24-26 min 10 % B; 26-28 min 10% B for a total runtime of 28 minutes. PDA was used to detect absorption at wavelengths between 190 nm to 800 nm.
Three independent extraction of Lobelia sample were used for HPLC-PDA analysis. The extracts were analysed using a Prominence-I liquid chromatograph (Shimadzu, MD, USA). Separation was achieved using an analytical reverse phase C18 column (Kinetex 5μm, 150mm×4.6 mm) (Phenomenex, ON, Canada) thermostated at 40°C. The solvents used were (A) 2.5 % acetic acid in water and (B) 100% acetonitrile. 20 μl of extract was injected and separated with the gradient method : 100 % A for 5 min, 98% A and 2% B for 2 min, 80% A and 20 % B for 2 min, 60% A and 40 % B for 15 min, 40% A and 60% B for 10 min, 20% A and 80% B for 10 min, 2% A and 98% B for 6 min and finally 98% A and 2% B for 4 min at a flow rate of 0.5 ml/ min. Photodiode Array Detector (PDA) in full scan mode was used to detect absorption at wavelengths between 190 nm to 800 nm.
Standards calibration curve
To evaluate the efficiency of the extraction method, a calibration curve was generated using piperine as an IS. Different concentrations of piperine ranging from 0 to 100 ppm were prepared in triplicate and analyze with the HPLC-PDA. The average (n=3) of the peak areas as a function of the concentration yielded a correlation coefficient of R² = 0,998 and the following linear regression equation: y = 109271x – 655460 which was used to quantify the IS recover after extraction (Figure 2B). The Limit of Detection (LOD) and of quantitation (LOQ) were calculated at 1 and 10 ppm, respectively.
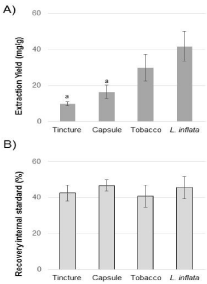
Figure 2: Extraction yield and reproducibility. L. inflata plant and supplement
samples were extracted using a liquid-liquid acid-based method. Values are
means ± standard deviation from three independent experiments. a above
the bars indicates mean values that are statistically different relative to the
L. inflata plants extract using Student’s t test: P < 0.05. A) Extraction yield
was determined by measuring the mass of compounds extracted over the
mass (or volume for tincture) of the starting material. B) Extraction efficiency
was measured using piperine as an internal standard. A ratio (expressed
in percent (%) recovery) between the quantity of piperine before and after
extraction is presented.
In addition, a calibration curve was created using the lobeline standard. Different concentrations ranging from 0,1 to 100 ppm (0,1, 0,5, 1, 5, 10, 15, 25, 35, 50, 75, 100 ppm) were prepared in triplicate and analyzed using HPLC. The average of the peak areas were plotted against lobeline concentrations to generate a graph with a correlation coefficient of R² = 0,997 and a linear regression equation of y = 54300x – 49408 which was used to quantified lobeline in the extracted samples.
LC-MS/MS Conditions
A Waters Alliance 2690 HPLC system coupled with Waters micromass Quattro LC Triple Quad Liquid Chromatography/Mass Spectrometer (LC-MS/MS) system was used to determine the exact m/z of the metabolites extracted in each sample. The separation was achieved using an analytical reverse phase C18 column (Kinetex 5μm, 100Å, 150mm×4.6 mm), the same column as the one used for the HPLC-DAD. Also, the same gradient elution was applied with 20 mM ammonium formate, pH 3 (solvent A) and acetonitrile 100% (solvent B). The separation was performed at a constant flow rate of 0.2 mL/ min with the following conditions : a linear gradient from 10% to 30% of B in 5 min; 5-32 min 30% B; 32-34 min 90% B; 34-36 min 90% B; 36-38 min 10 % B; 38-40 min 10% B. The column temperature has been set at 20°C (and not 40°C like the HPLC analysis because of the absence of a column oven). LC-MS/MS analyses were performed under a Positive Electrospray Ionization mode (+ESI) with the same method as for HPLC except the flow rate was reduced to 0.2 mL/min (to favor ionization and avoid electric arching). Also the LC-MS/MS runtime doubled to 40 min rather than 20 min for HPLC. In order to ensure the right detection of our targeted alkaloids, we used tandem Mass Spectrometry (MS/MS). During MS/MS analyses, all molecules of interest were characterized by the isolation of the parent molecules in Q1, the specific fractionation of the parent molecules in a collision cell at a selected energy in q, and finally the scan of the characteristic ions fragments in Q2. The conditions of the MS/MS section were set to acquire in positive ion mode as follows: desolvation gas flow rate 70 L/hr, desolvation gaz temperature 350°C, source temperature 120°C, capillary voltage 1.00 kV, cone voltage 30 V, scan mass range from 50 to 350 +ESI and collision energy of 20 V. Each sample previously analyzed by HPLC, was diluted by a factor 1000 to fit with the sensitivity of the LC-(+ESI)-MS/MS apparatus.
Results and Discussion
Extraction yield and efficiency
Lobelia inflata plants and Lobelia-derived products, including tobacco and supplements such as liquid tincture and capsules, were extracted with methanol. These crude extracts were fractionated using a liquid-liquid acid-base extraction method specifically targeting alkaloid compounds. The extraction yield was higher with L. inflata plant tissues compared to Lobelia supplements (Figure 2A). For example, L. inflata yielded the higher amount with 41.67 ± 8.22 mg/g whereas the tincture extract was significantly lower compared to all other extracts with 9.83 ± 1.11 mg/g. Since a tincture is basically an alcoholic extract, the tincture supplement has most likely already been extracted commercially prior to our acid-base extraction, which resulted in a significantly lower yield (Figure 2A). The yield of the capsule extract was significantly lower compared to L. inflata, but not compared to tobacco sample. Thus, the extraction method yield similar amount of extracted compounds for plant extracts including tissues and tobacco whereas supplements (tincture and capsule) yielded less compounds extracted.
The efficiency of our extraction method was measured using piperine as an Internal Standard (IS). Previous analysis confirmed the absence of piperine in all of the Lobelia samples. Thus, prior to the extraction, 250 ppm of piperine was added to each sample and was quantified in the end using HPLC analysis. Piperine peaks were quantified using peak area with the piperine calibration curve. Altogether, the extraction efficiency was similar (40-50% recovery) for all sample (Figure 2B) suggesting that our method was reproducible.
Thin Layer Chromatography (TLC) results
Next, the extracted compounds were visualized using chromatographic methods. Thin-Layer Chromatography (TLC) plates F254 containing a fluorophore that display a shadow under UV if compound is present were used. Thus, TLC were revealed under UV 254 nm, 365 nm and chemically using the Dragendorff reagent (Figure 3). This reagent was useful to quickly reveal compounds with heterocyclic amines such as alkaloids. Indeed, the interaction between the reagent and an amine group produces an orange colored complex. TLC showed qualitative differences among alkaloids from the different samples (Figure 3A). We found that all samples contained various unknown compounds. Plant extracts (L. inflata and tobacco) possessed larger number of various alkaloids in comparison to supplement extracts, including spots on TLC with Rf not corresponding to the lobeline standard (Figure 3). Tincture extracts appeared to contain less alkaloids than the other extracts. Thus, TLC analyses showed differences in the alkaloid profiles of the different sample extracted.
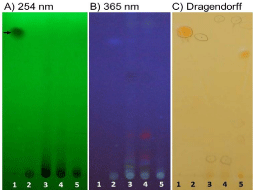
Figure 3: TLC pictures of extracted samples. TLC F254 contained a
fluorophore that display a shadow under UV if compound is present. Samples
are standard 1-lobeline and L. inflata extracts 2-tincture, 3-capsules,
4-tobacco and 5-L.inflata whole plants. TLC were revealed under 254 nm
light (A), 365 nm (B) and chemically using the Dragendorff’s reagent (C).
HPLC analysis of extracts
To quantify the differences in alkaloid profiles, High-Performance Liquid Chromatography (HPLC) analyses were performed and compounds were detected using Photodiode Array detector (PDA). Peaks for standards at specific Retention Time (Rt) and absorption spectrum (λmax) were detected specifically lobeline (Rt 15.3 min; λmax 246 and 211 nm) and piperine as IS (Rt 25.9 min; λmax 340 and 254 nm) (Figure 4). The IS piperine was detected in all sample as expected and was used to quantify extraction yield (Figure 2). In addition, a peak was detected with similar Rt and λmax for lobeline in capsule, tobacco and L. inflata whole plant extracts, whereas no lobeline peak was detected in the tincture extracts (Figure 4). Previous studies have shown the presence of lobeline in different tissues of L. inflata plants [7,12]. Using the lobeline standard calibration curve, we quantified the amount of lobeline per g of extracted sample. Higher concentration of lobeline were found in tobacco (17.64 μg/g) followed by whole L. inflata plant (0.95 μg/g) and capsule (0.77 μg/g) extracts. Since tobacco samples are dried leaves of plant, it is expected that the concentration of lobeline to be higher. Several peaks, with the same retentions times (Rt 2.8, 3.0, 7.4, 7.8, 8.1, and 9.8 min), were detected in all extracts suggesting a variety of compounds present in all samples. Also, specific peaks, only detected in a few sample were found. For example, peak with Rt 8.5 min was only detected in the plant extract, whereas peak Rt 5.8 min was only detected in the tincture extract (Figure 4). Interestingly, based on the area under the peaks, capsule and tobacco extracts had respectively 1.7 and 1.6 times more compounds compared to L. inflata plants extract, whereas tincture extract were 0.8 times less (Figure 4). These results correlated with TLC data where tincture extract showed the lowest amount of alkaloid extracted.
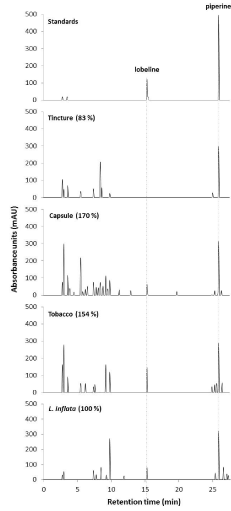
Figure 4: HPLC chromatograms of extracted L. inflata samples.
Representative HPLC chromatogram for known standards (top) including
lobeline and piperine and from extracts of different sample of L. inflata.
Absorbance at 280 nm in Milli-Absorbance Units (mAU) is a function of
the retention time (min). Number in parenthesis represents the relative
percentage (%) of total metabolites detected compared to L. inflata plant
extract normalized to 100%.
LC-MS/MS analysis of extracts
Next, extracted sample solutions were subjected to LC-MS/MS analysis using a Positive Electrospray Ionization mode (+ESI) with the same method as for HPLC except the flow that was reduced to 0.2 mL/min (to favor ionization and avoid electric arching) while the total method time doubled to 40 min rather than 20 min for HPLC. The QqQ dual +ESI source conditions were optimized using alkaloid standards to obtain a good signal and high sensitivity. The conditions such as capillary voltage, spray voltage and skimmer voltage were enhanced to maximize the ionization in the source and sensitivity to identify and characterize all possible degradation products. We observed predicted major mass spectral fragments for the IS piperine m/z 286 [M + H]+ at Rt 37.91 min (Table 1 & Supplementary data 2). Precisely, the MS ion fragment m/z 201 was assigned to a cleavage of the carboxyamide moity and its derived ions m/z 171 and m/z 143 (Supplementary data 1). Similar MS/MS mass spectra patterns were reported [13,14] suggesting a good fragmentation of our IS piperine.
Lobelia alkaloid Group
Retention Time (min)
[M+H]+
Proposed identification
ESI(+)-CID m/z
Reference CID
I
3.15
226
8-methyl-10-ethyllobelidione
168, 154
[16]
I
3,78
226
8-methyl-10-ethyllobelidione
168, 154
[16]
I
3.94
228
8-methyl-10-ethyllobelionol
210, 170, 156, 152
[16]
I
4,12
240
8,10-diethyllobelidione
168
[16]
I
5.37
240
8,10-diethyllobelidione
168
[16]
6.26
346
208, 164
I
7.26
242
8,10-diethyllobelionol
224, 170, 168, 152, 116
[16]
I
8.95
242
8,10-diethyllobelionol
224, 170, 168, 152, 116
[16]
II
11.65
206
norallosedamine
[16]
II
12.74
220
allosedamine
[16]
II
15.95
278
norlelobanidine
260, 202
[16]
II
17.32
306
8-ethyl-10-phenyl-hydroxylobelionol
288, 156, 140, 107
[16]
II
17.41
292
lelobanidine
274, 202, 170, 112
[16]
20.32
320
279, 214, 154, 105
II
23.55
276
8-ethyl- 10-phenyl-norlebelionol
258, 204, 186
[16]
III
24.00
340
lobelanidine
322, 218, 202, 114
[16]
III
24.69
338
lobeline
320, 218, 216, 200, 188, 105
this study
36.49
340
320, 198, 163
36.60
330
313, 289, 269, 207
37.53
348
325, 173, 113
37.91
286
piperine
201, 171, 159, 143, 135, 115, 112, 86
this study
38.15
304
212
III
38.24
322
norlobelanine
202
[16]
39.77
348
330, 249, 239, 185
III
39.92
322
norlobelanine
202
[16]
Table 1: Metabolite list obtained from L. inflata whole plant extract and their corresponding CID spectra by LC-MS/MS analysis.
In addition, the +ESI-MS/MS analysis of the lobeline standard revealed a Rt of 24.69 min for the molecular ion m/z 338 [M+H]+ with the majority of ion fragments of m/z 320, 218, 216, 200, 188 and 105 (Table 1 & Figure 5). The ion fragment m/z 320 was obtained through the loss of H2O from the molecular ion m/z 338. The ion at m/z 218 was formed by the elimination of a phenyl-2-ketoethyl side chain (120 Da) whereas m/z 216 was produced by loss of a phenyl- 2-hydroxyethyl moiety (122 Da) (Table 1 & Figure 5). The more abundant product ion at m/z 200 corresponded to the dehydratation of m/z 218 [218-18] (Figure 5). Previous MS/MS studies revealed that the fragmentation of the molecular ion of lobeline led to similar product ions [7,12] thereby validating the method for the subsequent MS/MS analysis of piperidine alkaloids from extracted sample.
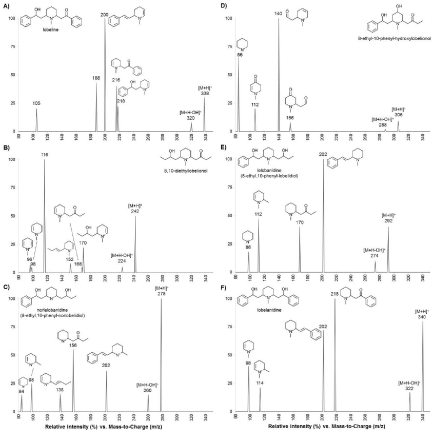
Figure 5: LC-MS/MS chromatograms. Characterization of piperidine alkaloids detected in Lobelia-derived products including the tincture, capsule and tobacco
extracts. Following liquid chromatography, parent ions were focused by Positive ion mode Electrospray Ionization (+ESI) mode and fragment ions were generated
by Collision-Induced Dissociation (CID). Characteristic fragment ions were analyzed in each +ESI-CID spectra are described. Possible chemical structures of major
mass spectral fragments are presented.
+ESI-MS/MS analyses were performed on extracts of L. inflata plant and of Lobelia-derived product primarily to deepen our HPLC data and to determine the major m/z detected in sample with the focus being on [M+H]+ molecular ions of even-masses indicative of single nitrogen-containing compounds including piperidine alkaloids. A total of 25 even-masses were detected in L. inflata plant extract (Table 1). Most masses corresponding to piperidine alkaloids were identified previously in aerial parts of L. inflata [7]. For instance, the Collision Induced Dissociation (CID) spectra of the molecular ion m/z 226 [M+H]+ in MS/MS product ions scan mode corresponded to 8-methyl-10-ethyllobelidione since it led to product ion m/z 168 from the elimination of a methyl-2-ketoethyl moiety (58 Da) and the ion m/z 154 from the loss of an ethyl-2-ketoethyl unit (72 Da) (Table 1 & Figure 1). Similarly, molecular ion m/z 228 [M+H]+ at Rt 3.94 min corresponded to 8-methyl-10-ethyllobelionol because it produced diagnostic fragment ions at m/z 170 from the loss of a methyl-2- ketoethyl residue (58 Da) and the ion m/z 210 from the elimination of the hydroxyl-group bearing side chain (Table 1 & Figure 1). Interestingly, molecular ion masses at Rt 11.65 and 12.74 minutes respectively corresponding to norallosedamine m/z 206 [M+H]+ and allosedamine m/z 206 [M+H]+ were also detected.
Among all masses identified in L. inflata plant extract, we screen for alkaloids present in the Lobelia-derived product extracts. Only unknown-14 at Rt 38.15 min and molecular ion m/z 304 was present in all three samples. However, the CID spectra did not allow for identification (Table 2). The CID spectra of unknown-3 (Rt 8.95 min, molecular ion m/z 242) led to its identification to 8,10-diethyllobelionol (Figure 5, Table 2) and showed that it was only present in the capsule and tobacco extracts. Norlelobanidine, 8-ethyl-10-phenyl-hydroxylobelionol, lelobanidine and lobelanidine were identified only in the capsule extract whereas unidentified unknown 10 and 11 were specific to the tincture extract (Figure 5, Table 2). Altogether, the results showed that few alkaloids could be detected from Lobelia-derived product. Since lobeline was not detected in the tincture extract compare to tobacco and capsule, it suggest possible difference of biological effects among Lobeliaderived natural product. It is possible that lobeline is modified or degraded during manufacturing of Lobelia-derived products leading to accumulation of others piperine alkaloids such as diethylobelionol and norlelobanidine with unknown biological activities.
Compound
Source Extract
[M+H]+
Retention Time (min)
Proposed identification
CE (eV)
+ESI-CID m/z (relative counts)
Lobeline
Authentic standard
338
24.69
20
338 (30), 320 (8), 218 (22), 216 (40), 200 (100), 188 (43), 105 (20)
Piperine
Authentic standard
286
37.91
20
286 (15), 201 (100), 171 (6), 159 (3), 143 (12), 135 (27), 115 (5), 112 (15), 86 (8)
Unknown-1
T
346
6.26
?
20
346 (8), 208 (100), 164 (33)
Unknown-2
C
242
7.26
8,10-diethyllobelionol
20
242(73), 224 (32), 170 (90), 168 (68), 152 (5), 116 (44), 98 (100), 96 (42)
Unknown-3
C, T
242
8.95
8,10-diethyllobelionol
20
242 (50), 224 (5), 170 (22), 168 (5), 152 (8), 116 (100), 98 (5), 96 (7)
Unknown-4
C
278
15.95
norlelobanidine
20
278 (100), 260 (15), 202 (36), 156 (55), 138 (16), 98 (25), 84 (15)
Unknown-5
C
306
17.32
8-ethyl-10-phenyl-hydroxylobelionol
20
306 (9), 288 (2), 156 (8), 140 (100), 107 (20), 84 (65)
Unknown-6
C
292
17.41
lelobanidine
20
292 (40), 274 (10), 202 (100), 170 (40), 112 (47), 98 (18)
Unknown-7
C
320
20.32
?
20
320 (38), 279 (15), 214 (18), 154 (100), 105 (20), 95 (26)
Unknown-8
C
340
24.00
lobelanidine
20
340 (80), 322 (17), 218 (100), 202 (72), 114 (21), 98 (40)
Unknown-9
C, T
338
24.69
lobeline
20
338 (30), 320 (8), 218 (22), 216 (40), 200 (100), 188 (43), 105 (20)
Unknown-10
L
340
36.49
?
20
340 (82), 320 (15), 198 (64), 163 (100)
Unknown-11
L
330
36.60
?
20
330 (11), 313 (16), 289 (25), 269 (100), 207 (20)
Unknown-12
T
348
37.53
?
20
348 (82), 325 (38), 173 (48), 113 (100), 91 (71)
Unknown-13
C, L, T
286
37.91
piperine (internal standard)
20
286 (15), 201 (100), 171 (6), 159 (3), 143 (12), 135 (27), 115 (5), 112 (15), 86 (8)
Unknown-14
C, L, T
304
38.15
?
20
304 (100), 212 (90), 91 (92)
Unknown-15
T
348
39.77
?
20
348 (5), 330 (42), 249 (27), 239 (100), 185 (28), 99 (31)
Table 2: Metabolite list obtained from Lobelia-derived products including Liquid tincture (L), Capsule (C) and Tobacco (T) extracts and their corresponding CID spectra by LC-MS/MS analysis.
In the course of our survey for alkaloids, a phytochemical study on L. inflata whole plants and plant-derived products led to the identification of specific piperidine alkaloids. Among them, lobeline and lobelanidine are known to exhibit biological properties. This study clearly shows interesting differences among the alkaloid profiles of Lobelia-derived product compared to whole plants. Compared to plants, the levels of lobeline, the main therapeutic metabolite of L. inflata, varies from not-detected (tincture) to similar (capsule) or higher (tobacco). This urges people to carefully be aware of the composition of natural products in supplement to justify/or not their selection for the treatment of ailment. For example, lobeline could have other effects than anti-addictive [15]. Herein, we report on the extraction and identification of major piperidine alkaloids from Lobelia plants and its derived products. For most of the newly identified alkaloids, their corresponding biological activity remains unknown. Therefore, the current pharmaceutical claims for Lobeliaderived commercial products has to be taken with care. Furthermore, the identification of potential metabolic precursors and intermediates in L. inflata paves the way to future studies aiming at elucidating the biosynthetic pathway of these valuable natural products.
Conclusion
The phytochemical investigation of L. inflata plants and of Lobelia derived-products with advanced mass spectrometry techniques led to the characterization of several piperidine alkaloids. Several molecular structure of alkaloids identified were confirmed via literature comparison and tandem mass spectrometry patterns. These compounds were reported for the first time in Lobelia-derived products, i.e. tincture and capsules. Owing to the pharmaceutical activity of piperine alkaloids, great care has to be taken when using these products. Furthermore, our study led to the identification of alkaloids in L. inflata plants, especially ones associated to potential metabolic precursors and intermediates. Thus, our study provides preliminary work supporting study on the biosynthetic pathway of piperidine alkaloid in plants. A better understanding of alkaloid metabolism and their biological activities could led the way to the development of new natural pharmaceuticals.
References
- USDA. United States Department of Agriculture: Natural Ressources Conservation Service. 2016.
- Moerman D. Native American Ethnobotany: A Database of Foods, Drugs, Dyes and Fibers of Native American Peoples. Derived from Plants. 2003.
- Crooks PA, Zheng G, Vartak AP, Culver JP, Zheng F, Horton DB, et al. Design, synthesis and interaction at the vesicular monoamine transporter-2 of lobeline analogs: potential pharmacotherapies for the treatment of psychostimulant abuse. Curr Top Med Chem. 2011; 11: 1103-1127.
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002; 63: 89-98.
- Chen MW, Chen WR, Zhang JM, Long XY, Wang YT. Lobelia chinensis: chemical constituents and anticancer activity perspective. Chinese Journal of Natural Medicines. 2014; 12: 103-107.
- Felpin FX, Lebreton J. History, chemistry and biology of alkaloids from Lobelia inflata. Tetrahedron. 2004; 60: 10127-10153.
- Kursinszki L, Szoke E. HPLC-ESI-MS/MS of brain neurotransmitter modulator lobeline and related piperidine alkaloids in Lobelia inflata L. J Mass Spectrom. 2015; 50: 727-733.
- Gupta RN, Spenser ID. The biosynthesis of sedamine. Canadian Journal of Chemistry. 1967; 45: 1275-1285.
- O’Donovan DG, Keogh MF. Biosynthesis of piperidine alkaloids. Tetrahedron Letters. 1968; 9: 265-267.
- Keogh MF, O’Donovan DG. Biosynthesis of lobeline. Journal of the Chemical Society C: Organic. 1970.
- Robles MA. [Modified Dragendorff reagent for the procurement of lasting coloration of spots in paper chromatographic and electrophoretic investigation of alkaloids]. Pharm Weekbl. 1959; 94: 178-179.
- Kursinszki L, Ludányi K, Szõke É. LC-DAD and LC-MS-MS Analysis of Piperidine Alkaloids of Lobelia inflata L. (In Vitro and In Vivo). Chromatographia. 2008; 68: 27-33.
- Kotte SCB, Dubey PK, Murali PM. Identification and characterization of stress degradation products of piperine and profiling of a black pepper (Piper nigrum L.) extract using LC/Q-TOF-dual ESI-MS. Anal Methods. 2014; 6: 8022-8029.
- Chithra S, Jasim B, Anisha C, Mathew J, Radhakrishnan EK. LC-MS/MS Based Identification of Piperine Production by Endophytic Mycosphaerella sp. PF13 from Piper nigrum. Applied Biochemistry and Biotechnology. 2014; 173: 30-35.
- Tamboli AM, Rub RA, Ghosh P, Bodhankar SL. Antiepileptic activity of lobeline isolated from the leaf of Lobelia nicotianaefolia and its effect on brain GABA level in mice. Asian Pacific Journal of Tropical Biomedicine. 2012; 2: 537-542.