
Review Article
Austin Biochem. 2018; 3(1): 1015.
Differential Impact of the Ubiquitin-Proteasome System on Clear Cell Carcinoma and High-Grade Serous Ovarian Cancer
Werts S and Ye J*
¹Department of Biochemistry, Salem College, USA
*Corresponding author: Ye J, Department of Biochemistry, Salem College, 601 S Church St, Winston- Salem, NC, 27101, USA
Received: April 04, 2018; Accepted: April 23, 2018; Published: April 30, 2018
Abstract
Ovarian cancer is particularly deadly and difficult to target because of its aggressive nature. With high mortality rates, current studies are focused on improving early detection and prevention methods as well as developing new treatments. To successfully develop these therapeutic options, researchers first need to understand how the cancer forms, spreads, and functions. Since ovarian cancer is composed of many heterogeneous subtypes, such as high-grade serous ovarian cancer and clear cell epithelial ovarian cancer, the nuances of each type need also be examined and understood in order to target each individually. It has been determined that each subtype has a unique relationship with the tumor suppressor, p53. Understanding how each subtype interacts with p53 can lead to specialized treatment mechanisms that target these interactions. MDM2 and ADRM1, over expressed in clear cell carcinoma and high grade serous ovarian cancer respectfully, are key components of these interactions within ovarian cancer. MDM2 is a negative regulator of p53, while ADRM1 aids in protein degradation. The overexpression of each molecule acts specifically to aid ovarian cancer in survival. In this review, recent advances in studying ovarian cancer subtypes will be covered, as well as how these subtypes relate to the ubiquitin-proteasome system and the key tumor suppressor in human cells, p53.
Keywords: Ubiquitin-proteasome system; p53; Clear cell carcinoma; Highgrade serous ovarian cancer
Abbreviations
DUBs: Deubiquitinating Enzymes; HGSC: High-Grade Serous Ovarian Cancer; CCC: Clear Cell Carcinoma; MDM2: Murine Double Minute 2; UPS: Ubiquitin-Proteasome System; ROCA: Risk Of Ovarian Cancer Algorithm; CIC: Cancer Initiating Cell; BRCA: Breast Cancer Gene; ADRM1: Adhesion Regulating Molecule 1; CA- 125: Serum Cancer Antigen-125
Introduction
Ovarian cancer, one of the deadliest and most aggressive gynecological malignancies, is the fifth most common cause of cancer death in women [1,2]. As recently as 2017, the annual ovarian cancer mortality was approximately 65% of the incidence rate due to low predictive value in screening procedures for women without increased risk factors [3]. As a result, only 15% of patients are diagnosed with localized disease and many patients are diagnosed in stage III or IV [1]. The four main histological subtypes within epithelial ovarian cancer are serous, clear cell, endometrioid, and mucinous adenocarcinomas [2,4,5]. Each subtype has its own unique abnormalities, making diagnosis and treatment of ovarian cancer difficult. While all women are susceptible to ovarian cancer, increased risk factors include familial history, nulliparity, lack of breast feeding, and infertility [1]. About 20% of ovarian cancers are familial, linked mostly to Breast Cancer Alleles 1 and 2, (BRCA1 or BRCA2), though other gene mutations have been implicated as well [3]. BRCA1 and BRCA2 produce tumor suppressor proteins and mutations in these genes and are commonly found in breast cancer as well as ovarian cancer. The overall five-year survival rate for ovarian cancer patients has improved over the last decade due to improvements in general cancer treatment methods, but survival for advanced stages is still less than 40%. Research in ovarian cancer is currently focused on defining the mechanism of formation and spread of each subtype, improving early detection and prevention, and creating new therapeutic options [1]. The goal of this review is to highlight current research on serous and clear cell epithelial ovarian cancer and determine if the ubiquitinproteasome system can be targeted in each cancer to create new screening tests or treatment options.
One of the most important proteins in regard to tumor suppression in humans is p53. Wild-type p53, a component of the ubiquitin-proteasome system, is activated by cellular stress and inhibits cell cycle progression, prompts apoptosis, or stimulates senescence [6]. In many human cancers, p53 is inactivated, either through mutation or other mechanism [7]. This allows tumor growth to continue unchecked. In some ovarian cancer subtypes, such as high-grade serous ovarian cancer, the p53 protein is mutated or deleted, and in some, such as clear cell carcinoma, the wild-type p53 protein is inactivated due to interaction with other molecules [8,9]. Inactivation of p53 can occur through interactions with various molecules within the ubiquitin-proteasome system, most commonly MDM2 and other Deubiquitinating Enzymes (DUBs) [10-13].
These molecules destabilize p53, making it inert and no longer able to regulate tumor growth. Targeting the pathways that lead to p53 inactivation, such as inhibitors of the MDM2-p53 interaction, could be beneficial to improving treatments for the advanced stages of ovarian cancer [11,12]. Each subtype of ovarian cancer has a link to p53. Understanding which mechanism blocks p53 in each subtype and how to target that mechanism can lead to specialized treatment and increased survival for ovarian cancer.
Comparison of CCC and HGSC
Understanding the subtype of ovarian carcinoma that is present can help lead physicians toward specialized treatment plans for each patient. Each histological subtype has unique molecular abnormalities, separate risk factors, and differing treatment needs [5,14-16]. The two main subtypes of ovarian cancer, based on frequency and mortality, are High-Grade Serous Ovarian Cancer (HGSC) and Clear Cell Carcinoma (CCC). HGSC is the most aggressive subtype of the disease. As recently as 2017, HGSC accounted for approximately 70% of epithelial ovarian cancer cases, but had a 5-year survival rate that was less than 40% [1,2,4,17]. CCC accounted for approximately 10% of ovarian cancer cases overall and as much as 25% of cases in Asia [14]. CCC is notoriously hard to target, especially if diagnosed in the advanced stages, and the 3-year survival rate was only about 10% of the confirmed cases in 2016 [18,19]. If diagnosed in stage III-IV, CCC can be very difficult to treat, accounting for low survival.
Each form has unique precursor lesions and sites of origin. Until recently, it was thought that all subtypes arose from ovarian surface epithelium, but new evidence shows that each type originates from a different non-ovarian tissue [2]. HGSC may originate from precursor lesions in the fimbriae of the fallopian tubes [16,20]. This is supported by evidence that risk-reducing surgeries lower the prevalence of disease in healthy women with BRCA1 and BRCA2 mutations [3]. Whole-exome sequencing of HGSC patients also supports this conclusion [21]. CCC can arise in the ovary, endometrium, and the cervix and has been linked to endometriosis in many studies [3,5,15,22-24]. HGSC mitotic rate is high and most patients will present at high-stage, with advanced metastasis, while CCC is usually low-stage and mitoses are less frequent [14,25,26]. Patients with CCC are also younger than patients with HGSC on average [25]. These differences arise from differences in the biochemistry of each subtype.
Molecularly, the two subtypes have different mutations. HGSC cases have defects in BRCA1, BRCA2, and TP53, while CCC cases frequently lack these mutations [14,23]. CCC patients commonly have mutations in ARID1A and PI3K, which are two genes involved with chromosome remodeling and cell growth and division [5,19,26,27]. MDM2 (Murine Double Minute 2), a ubiquitin ligase, has significantly higher expression in CCC than in HGSC. This overexpression of MDM2 blocks wild-type p53 function, allowing cancerous cell growth to continue uninhibited and leads to poor overall survival in CCC [18,25]. In HGSC, ADRM1, which encodes part of the 19S regulatory particle, is over expressed. Overexpression of ADRM1 is important for HGSC survival because it helps recognize and degrade the excess polyubiquitinated proteins produced by the cancer, allowing it to continue creating more proteins as it rapidly metastasizes [28]. These pathways and how they may be targeted are explained in further detail below.
Biochemical Pathways
MDM2 and p53
Dysfunction of p53 has important implications for both HGSC and CCC, though each is caused by a different mechanism. In HGSC, p53 does not function to regulate tumor growth due to the TP53 mutation seen in greater than 90% of HGSC cases [1,5,29,30]. In CCC, patients have a wild-type TP53 gene, but normal function is inhibited by DUB molecules. Recent evidence has shown that ovarian cancer patients without TP53 mutation were likely to have p53 dysfunction associated with copies of the E3 ubiquitin ligase MDM2 [7]. MDM2 plays a crucial role in the degradation and regulation of p53 in the body. In normal cells, wild-type p53 is short lived and acts to suppress tumor formation and inhibit cell growth [31,32]. It can bind to specific DNA sequences in order to activate the transcription of genes involved with stress and tumor suppression. One of the genes that p53 binds to is the MDM2 gene. The p53 protein can bind to the MDM2 gene in order to regulate its level of transcription, while MDM2 can in return bind to p53 to regulate its level of activity in the body [33,34]. Generally, MDM2 activity will be seen an hour after p53 in order to slow down the p53 response. MDM2 acts as a negative regulator for p53, inhibiting and suppressing p53 function via proteasome-mediated degradation and terminating the p53 signal in normally functioning cells [35,36]. These molecules are tightly controlled and inversely correlated; when MDM2 levels rise, p53 levels lower [18,31]. In certain cancers, MDM2 overexpression creates such a strong negative feedback signal that it inhibits normal function of p53 (Figure 1). This overexpression occurs in CCC ovarian cancer.
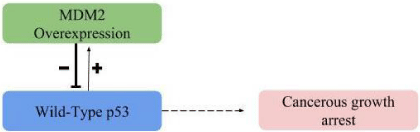
Figure 1: MDM2 overexpression in CCC.
In a study by Makii et al., it was discovered that MDM2 expression is significantly higher in CCC than in HGSC and that this overexpression was correlated to poor prognosis in CCC patients [18]. Overexpression may be correlated with the chemoresistance seen in CCC cases and therefore the poor outcome for CCC patients. This could prompt further research into MDM2 inhibitors as a treatment for ovarian cancer [18,29]. Currently, MDM2 is not used as a diagnostic factor when screening for ovarian cancer or CCC, but it may be beneficial to use in the future. A possible reason that there is variation in CCC rates in eastern countries versus western countries is that MDM2 has different haplotypes, one which is linked to higher cancer risk and another which shows protective benefit. The haplotype that confers protection decreases the binding of the Sp1 transcription factor while the haplotype associated with cancer risk enhances the binding. The protective haplotype is only seen in Caucasians, which may account for why CCC rates are generally higher in Asian populations [37]. MDM2 is one of the most important molecules for p53 regulation. As the concentration of MDM2 increases, the ability for the wild-type p53 molecule to function decreases. If the autoregulatory feedback loop between these two molecules is mutated or abnormal, atypical cell growth will occur.
ADRM1 and the UPS
In a typical human cell, the Ubiquitin-Proteasome System (UPS) will mark and degrade damaged or abnormal proteins [38]. Ovarian cancers are characterized by an abundance of misfolded proteins caused by the rapid mitotic development of ovarian cancer tumors. This abundance of proteins in the cytosol of the cell necessitates a reliance on the UPS to degrade the excess protein before it becomes toxic to the cancer cells. Due to this reliance on the UPS, research has begun to target proteasome inhibitors, but the current proteasome inhibitor, bortezomib, has shown little efficacy [10]. Recently, RPN13 has been looked into as a potential new target. ADRM1 is a gene that encodes RPN13, which is a ubiquitin receptor tied to the 19S particle of a proteasome [28]. RPN13 recognizes and degrades ubiquitinated proteins, helping to increase the amount of misfolded proteins that can be broken down in the cell (Figure 2). In HGSC, ADRM1 is over expressed, which is correlated with stage III-IV ovarian cancer, shorter time to cancer recurrence and lower survival, signifying a possible correlation between RPN13 and disease progression. This overexpression has also been shown to increase cell division and growth, leading researchers to classify ADRM1 as an oncogene and potential target for future research [39]. The specific pathways affected by ADRM1 have yet to be determined, but it is thought that overexpression of ADRM1 mRNA may be an early event, found even in ovarian cancer precursor lesions to accommodate excess misfolded proteins [28]. RPN13 may be an important protein to look for during screening or to target during treatment.
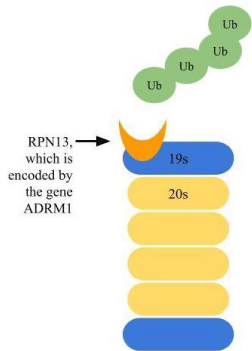
Figure 2: ADRM1 overexpression in HGSC.
Screening and Diagnosis
There are currently no approved screening tests for early detection available for women without an increased risk of ovarian cancer. One of the main reasons that ovarian cancer is commonly diagnosed in stage III or IV is because there is not a feasible test available to detect it early and the cancer is relatively rare in the population, so the symptoms will generally go unnoticed. Women are told to check their body for any abnormal changes and monitor for the symptoms. Symptoms, however, are vague and offer little detail.
Back pain, bloating, pelvic pain, and constipation are a few examples of what women are supposed to monitor, but often these changes go undetected or are attributed to stress [40,41]. One study has shown that 89% of women diagnosed with stage I-II disease reported these symptoms before being diagnosed and 97% of women diagnosed with stage III-IV reported them [42]. Some women experience symptoms up to one year before they are diagnosed with ovarian cancer [43]. Another study showed that only 68% of patients declared that the symptoms prompted them to seek diagnosis [40]. This shows that the symptoms may not be enough to target ovarian cancer until it is too late. Some call ovarian cancer the “silent killer”, but it is not silent, it is just ignored [43]. These symptoms need to be better clarified or emphasized so women understand what they are looking for and can communicate these with medical professionals early.
Screening women who are at an increased risk of ovarian cancer is currently the most reasonable and practical option. This narrows the pool of women who are taking the screening tests and also offers a target for who physicians should be monitoring as their risk increases. One reason this is the best option currently is because many screening tests are not 100% accurate. Large randomized controlled studies have looked into using a combination of serum markers, such as serum cancer antigen-125 (CA-125), along with ultrasound imaging. CA-125 may be elevated in other disorders, such as endometriosis, so using it alone is not as effective to indicate ovarian cancer [3]. Though these have shown some benefit in high risk women, they also have a high-rate of false positives or inadequate sensitivity, resulting in 65-97% of women receiving unnecessary surgical procedures [1,44]. Multimodal screening tests are considered to be the best for early screening currently [45]. There is some controversy over the effectiveness of these tests and whether or not they should be put into place, because of the low sensitivity. The Risk of Ovarian Cancer Algorithm (ROCA) is based on CA-125 levels in the body and risk is assigned using a baseline and how these levels change over time [46]. This test may be most beneficial in high-risk women, where the benefits outweigh the costs. Other new screening options are currently in clinical trial. One in particular, which encourages riskreducing salpingo oophorectomy, is looking promising for detecting and lowering the rate of cancer early in patients with BRCA mutations [44]. Though a screening test that could be used in the general population would be nice, it is unlikely that a test could be developed that is cost effective and highly sensitive. Ovarian cancer is generally rare in the population and these tests may result in unnecessary intervention.
Future Treatments
Though different subtypes of ovarian cancer have different molecular abnormalities, each is treated as if they were the same. Despite their differences, each is treated with the treatment that currently works for HGSC. A platinum and taxane-based chemotherapy is the most commonly used treatment for all subtypes [5]. One reason CCCs can have a poor prognosis is because they do not respond well to treatment. CCCs are typically resistant to platinumbased chemotherapy and therefore respond less favorably to these treatment methods than HGSC [14,18,19]. Recent experimentation has shown that CCC response to chemotherapy was only 25%, compared to 73% in HGSC [24]. Individualized treatments need to be developed to target each subtype of ovarian cancer.
Cancer cells depend on the UPS system more than normal cells so new treatments should focus on targeting and inhibiting the proteasome [47]. Research is currently looking into ways to target MDM2 inhibitors, such as RG7112, to improve outcome for these patients [18]. Another treatment that is currently in clinical trial is an MDM2 inhibitor called Nutlin-3a. This inhibitor is dependent on p53 mutation status and may work well in CCC, where p53 is wildtype [5]. Both RG7112 and Nutlin-3a act as MDM2 antagonists, inhibiting the interaction between MDM2 and p53 and allowing for stabilization of p53 [18]. Blocking the overexpression of MDM2 in CCC would potentially restore p53 function, allowing for apoptosis of cancer cells and tumor suppression.
HGSC is more responsive to chemotherapy, but some patients may develop chemotherapy resistance over time [1]. Approximately 75% of HGSC cases respond to initial treatment but experience recurrence and eventually succumb to the disease [48]. One reason may be that the commonly used platinum-based therapies are able to kill differentiated cells, but not Cancer Initiating Cells (CICs), which can self-renew indefinitely without differentiating. The progeny of these cells are then able to differentiate and invade the body while evolving resistance to chemotherapy and drug treatments [49]. In patients with overexpression of ADRM1, the proteasome inhibitor RA190, targeting at RPN13, may be a potential solution. HGSC cells have shown sensitivity to RA190, which blocks RPN13 and inhibits proteasome function [28,50]. This will stop cancer cells from degrading misfolded proteins, causing a toxic build-up of proteins within the cytosol. Prophylactic oophorectomy reduces the risk of ovarian cancer by over 90% in women who are deemed to be at an increased risk based on family history [3]. Preoperative images can predict pathological subtype and better predict behavior of the carcinoma in order to choose a more individualized treatment that is more effective for the patient [48].
Conclusions
Ovarian cancer subtype holds important implications for diagnosis and treatment. Understanding how different subtypes work and evolve is crucial for creating treatments that are most effective at targeting and curing each different type. The two most common subtypes of ovarian cancer, HGSC and CCC, both have very different molecular abnormalities but are linked to the UPS. CCC shows overexpression of MDM2, which suppresses wild-type function of p53. HGSC shows overexpression of ADRM1, which increases the rate of recognition and degradation of polyubiquitinated proteins that ovarian cancer cells produce. If a screening or diagnostic test can be developed to measure this early overexpression in each molecule, then maybe these tests could be specified to disease and subtype with lower false-positive rates. Treatments are currently being tested to target and inhibit the proteasome, which would inhibit the overexpression of both of these molecules and ideally return the cell to normal tumor suppression or promote apoptosis due to toxic protein build up. This field is still developing, but if researchers can understand and harness the UPS in ovarian cancer, these tactics would have implications in many human cancers.
Normally, p53 and MDM2 act in a feedback loop with each other, with MDM2 regulating p53 levels as it monitors atypical cell growth. When MDM2 is overexpressed, it inhibits wild-type p53 ability to function. This stops tumor suppression and allows cancer cell growth to continue unchecked by p53.
Normally, RPN13 is able to recognize polyubiquitinated proteins and mediate protein degradation. In HGSC, RPN13 is overexpressed, allowing for large amount of misfolded proteins to be recognized and degraded.
References
- Mills K, Fuh K. Recent Advances in Understanding, Diagnosing, and Treating Ovarian Cancer. F1000Res. 2017; 6: 84.
- Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013; 4.
- Board PATE. Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD). 2002.
- Xie H, Wang W, Sun F, Deng K, Lu X, Liu H, et al. Proteomics analysis to reveal biological pathways and predictive proteins in the survival of highgrade serous ovarian cancer. Sci Rep. 2017; 7: 98-96.
- Crane EK, Kwan SY, Izaguirre DI, Tsang YT, Mullany LK, Zu Z, et al. Nutlin-3a: A Potential Therapeutic Opportunity for TP53 Wild-Type Ovarian Carcinomas. Plos One. 2015; 10.
- Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012; 26: 1268-1286.
- Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010; 221: 49-56.
- Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao F, et al. Integrated genomic analysesof ovarian carcinoma. Nature. 2011; 474: 609-615.
- Walton J, Blagih J, Ennis D, Leung E, Dowson S, Farquharson M, et al. CRISPR/Cas9-Mediated Trp53 and Brca2 Knockout to Generate Improved Murine Models of Ovarian High-Grade Serous Carcinoma. Cancer Res. 2016; 76: 6118-6129.
- Devine T, Dai MS. Targeting the ubiquitin-mediated proteasome degradation of p53 for cancer therapy. Curr Pharm Des. 2013; 19: 3248-3262.
- Shangary S, Wang SM. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008; 14: 5318-5324.
- Wang SM, Zhao YJ, Aguilar A, Bernard D, Yang CY. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Csh Perspect Med. 2017; 7.
- Zhao YJ, Aguilar A, Bernard D, Wang SM. Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction (MDM2 Inhibitors) in Clinical Trials for Cancer Treatment. J Med Chem. 2015; 58: 1038-1052.
- Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009; 40: 1213-1223.
- Winterhoff B, Hamidi H, Wang C, Kalli KR, Fridley BL, Dering J, et al. Molecular classification of high grade endometrioid and clear cell ovarian cancer using TCGA gene expression signatures. Gynecol Oncol. 2016; 141: 95-100.
- Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposedunifying theory. Am J Surg Pathol. 2010; 34: 433-443.
- Lengyel E, Burdette JE, Kenny HA, Matei D, Pilrose J, Haluska P, et al. Epithelial ovarian cancer experimental models. Oncogene. 2014; 33: 3619- 3633.
- Makii C, Oda K, Ikeda Y, Sone K, Hasegawa K, Uehara Y, et al. MDM2 is a potentialtherapeutic target and prognostic factor for ovarian clear cell carcinomas with wild type TP53. Oncotarget. 2016; 7: 75328-753238.
- Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015; 6: 6118-7118.
- Zeppernick F, Meinhold-Heerlein I, Shih Ie M. Precursors of ovarian cancer in the fallopiantube: serous tubal intraepithelial carcinoma--an update. J Obstet Gynaecol Res. 2015; 41: 6-11.
- Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017; 8: 1093.
- Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012; 13: 385-94.
- Grandi G, Toss A, Cortesi L, Botticelli L, Volpe A, Cagnacci A. The Association between Endometriomas and Ovarian Cancer: Preventive Effect of Inhibiting Ovulation and Menstruation during Reproductive Life. Biomed Res Int. 2015.
- Glasspool RM, McNeish IA. Clear cell carcinoma of ovary and uterus. Curr Oncol Rep. 2013; 15: 566-572.
- Ye S, Yang JX, You Y, Cao DY, Huang HF, Wu M, et al. Comparison of Clinical Characteristic and Prognosis between Ovarian Clear Cell Carcinoma and Serous Carcinoma: A 10-Year Cohort Study of Chinese Patients. Plos One. 2015; 10.
- Ku FC, Wu RC, Yang LY, Tang YH, Chang WY, Yang JE, et al. Clear cell carcinomas of the ovary have poorer outcomes compared with serous carcinomas: Results from a single-center Taiwanese study. J Formos Med Assoc. 2017.
- Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007; 11: 321-333.
- Jiang RT, Yemelyanova A, Xing D, Anchoori RK, Hamazaki J, Murata S, et al. Early andconsistent overexpression of ADRM1 in ovarian high-grade serous carcinoma. J Ovarian Res. 2017; 10: 53.
- Yang-Hartwich Y, Soteras MG, Lin ZP, Holmberg J, Sumi N, Craveiro V, et al. p53 protein aggregation promotes platinum resistance in ovarian cancer. Oncogene. 2015; 34: 3605-3616.
- Wong KK, Izaguirre DI, Kwan SY, King ER, Deavers MT, Sood AK, et al. Poor survival with wild-type TP53 ovarian cancer? Gynecol Oncol. 2013; 130: 565-569.
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997; 387: 299-303.
- Zhang R, Wu X, Xia X, Khanniche A, Song F, Zhang B, et al. OVA12 promotes tumor growth by regulating p53 expression in human cancer cells. Oncotarget. 2017; 8: 52854-52865.
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. GenesDev. 1993; 7: 1126-1132.
- Cheng TH, Cohen SN. Human MDM2 isoforms translated differentially on constitutive versus p53-regulated transcripts have distinct functions in the p53/MDM2 and TSG101/MDM2 feedback control loops. Mol Cell Biol. 2007; 27: 111-119.
- Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013; 13: 83-96.
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997; 387: 296-299.
- Knappskog S, Bjornslett M, Myklebust LM, Huijts PE, Vreeswijk MP, Edvardsen H, et al. The MDM2 promoter SNP285C/309G haplotype diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer Cell. 2011; 19: 273-282.
- Yang JM. Emerging roles of deubiquitinating enzymes in human cancer. Acta Pharmacol Sin. 2007; 28: 1325-1330.
- Fejzo MS, Anderson L, von Euw EM, Kalous O, Avliyakulov NK, Haykinson MJ, et al. Amplification Target ADRM1: Role as an Oncogene and Therapeutic Target for Ovarian Cancer. Int J Mol Sci. 2013; 14: 3094-3109.
- Olson SH, Mignone L, Nakraseive C, Caputo TA, Barakat RR, Harlap S. Symptoms of ovarian cancer. Obstet Gynecol. 2001; 98: 212-217.
- Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG. 2005; 112: 857-865.
- Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004; 291: 2705-2712.
- Friedman GD, Skilling JS, Udaltsova NV, Smith LH. Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract. 2005; 22: 548-553.
- Berchuck A, Havrilesky LJ, Kauff ND. Is There a Role for Ovarian Cancer Screening in High-Risk Women? J Clin Oncol. 2017; 35: 1384-1386.
- Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016; 387: 945-956.
- Rosenthal AN, Fraser LSM, Philpott S, Manchanda R, Burnell M, Badman P, et al. Evidenceof Stage Shift in Women Diagnosed With Ovarian Cancer During Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017; 35: 1411-1420.
- Huang HB, Liu N, Liao YN, Liu NN, Cai JY, Xia XH, et al. Platinum-containing compoundplatinum pyrithione suppresses ovarian tumor proliferation through proteasome inhibition. J Exp Clin Canc Res. 2017; 36: 79.
- Ohsuga T, Yamaguchi K, Kido A, Murakami R, Abiko K, Hamanishi J, et al. Distinct preoperative clinical features predict four histopathological subtypes of high-grade serouscarcinoma of the ovary, fallopian tube, and peritoneum. BMC Cancer. 2017; 17: 580.
- Nagaraj AB, Joseph P, Kovalenko O, Singh S, Armstrong A, Redline R, et al. Critical role of Wnt/beta-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015; 6: 23720-23734.
- Song Y, Ray A, Li S, Das DS, Tai YT, Carrasco RD, et al. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia. 2016; 30: 1877-1886.