
Reserach Article
Austin Biochem. 2018; 3(1): 1017.
Protective Effect of Palmitoleic, Oleic, and Vaccenic Acid on Structure -Function of Major Antioxidant Enzymes: Catalase, Superoxide Dismutase and Glutathione Peroxidase in the Hyperglycemic Environment: an In Vitro Study
Mirmiranpour H¹, Rabizadeh S¹, Mansournia MA², Salehi SS¹, Esteghamati A¹ and Nakhjavani M¹*
¹Department of Endocrinology and Metabolism Research Center (EMRC), Tehran University of Medical Sciences, Iran
²Department of Epidemiology and Biostatistics, Tehran University of Medical Sciences, Iran
*Corresponding author: Manouchehr Nakhjavani, Department of Endocrinology and Metabolism Research Center (EMRC), Vali-Asr Hospital, Tehran University of Medical Sciences, Tehran, Iran
Received: May 04, 2018; Accepted: June 08, 2018; Published: June 15, 2018
Abstract
Antioxidant enzymes are necessary for cellular viability in hyperglycemia. The aim of this study was to assess changes in structure and function of antioxidant enzymes in interaction with glucose and unsaturated fatty acids. The field of our study is basic sciences and in vitro experiments.
Each enzyme includingcatalase, SOD (Superoxide Dismutase) and GPx (Glutathione Peroxidase), in the present and absent of glucose were incubated for 4 months with and without fatty acids including palmitoleic acid, oleic acid, and vaccenic acid separately. Enzymes were assessed for fluorescence emission, Circular Dichroism (CD) and activity every 14 days.
Results showed that all three enzymes had asignificant increase in fluorescence emission (p<0.001) and adecrease in activity (p<=0.04) and significant change in CD (except CD in GPx) after incubation with glucose over time. Catalase and SOD after incubation with glucose and each of the fatty acids had less increase in fluorescence emission and significant change in CD toward normal compared to incubation of these enzymes with glucose alone(P<0.05). But GPx had no significant change. This study showed the protective role of nonessential unsaturated fatty acids against structural damage to catalase and SOD in the hyperglycemic environment GPx had different behavior.
Keywords: Antioxidant enzymes; Non-Essential unsaturated fatty acids; Glycation damage; in vitro Hyperglycemia
Abbreviations
SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; CD: Circular Dichroism
Introduction
Oxidative stress has an important role in cellular damage due to hyperglycemia [1]. There are a large number of antioxidants in cells to prevent damage from Reactive Oxygen Spices (ROS) including superoxide and hydrogen peroxide which react with proteins, lipids and DNA [2]. Antioxidant enzymes that are necessary for life in cells are Superoxide Dismutase (SOD), catalase and glutathione peroxidase. SOD can catalyze superoxide into hydrogen peroxide and oxygen. It has an important role in protection against ROS-induced cellular damage [3]. Down regulation of renal SOD may be important in the pathogenesis of diabetic nephropathy [1]. Catalase is an antioxidant enzyme that has an important role in protection against oxidative stress generated complications in diabetes. Catalase can convert hydrogen peroxide to oxygen and water [4]. It can prevent pancreatic beta cell damage due to hydrogen peroxide [5]. Catalase deficiency may be lead- to oxidative damage to pancreatic beta cells [4].
Glutathione Peroxidase (GPX), a selenoprotein enzyme, can convert hydrogen peroxide to water. This enzyme protects cells from oxidative stress [6,7]. Secondary enzymes such as glutathione reductase and glucose 6 phosphate dehydrogenase and several cofactors are necessary for its function [8]. In glycation conditions, antioxidant enzymes suffer from glycation damage same as other proteins. Catalase, Glutathione peroxidase, and SOD are enzymes that suffer from glycation damage and experience changes in their structures and function [9-11]. But in non glycation conditions, antioxidant enzymes can protect proteins from oxidative stress [12]. antioxidative effect of polyunsaturated fatty acids and their direct relation to increasing activity of antioxidant enzymes have been reported [13,14]. Also relationships between the decrease of cardiovascular events and consumption of unsaturated fatty acids have been reported [15,16]. Fish oil, a polyunsaturated fatty acid, can increase the activity of antioxidant enzymes such as catalase, glutathione peroxidase and superoxide dismutase [17]. Oleic acid and palmitoleic acid are monounsaturated fatty acids [18]. A meta-analysis of randomized controlled trials revealed that high monounsaturated fatty acid diet can improve metabolic risk factors in type2 diabetes [19]. A case-control study in 2016 showed aninverse association between monounsaturated fatty acids and oleic acid intake with diabetic retinopathy [20].
The aim of this study was to research the protective effect of monounsaturated fatty acids upon antioxidant enzymes against glycation damage in the hyperglycemic environment. We investigated changes in structure and function of these enzymes in the hyperglycemic condition in the absence and presence of each of the unsaturated nonessential fatty acids including palmitoleic, oleic and vaccenic acid in vitro.
Material and Methods
Materials
Antioxidant enzymatic proteins including catalase (C1345), glutathione peroxidase (G4013), and superoxide dismutase (S9636); unsaturated nonessential fatty acids including palmitoleic acid (P9417), oleic acid (O1008) and cis-vaccenic acid (V0384); glucose (G7021) and also phosphate buffered saline (PBS) (P5368) were purchased from Sigma Company (USA). 0.22 μm filter was purchased from Millipore Corporation, Billerica, MA (USA).
Methods
Glycation of antioxidant enzymes: Solution of the pure material of each enzyme, as the concentration of 10 mg/ml, was made by combining each enzyme with Phosphate Buffered Saline (PBS) at pH 7.4. Glucose solution was prepared by combining pure glucose with PBS. Then, a sample of pure enzyme solution was mixed with glucose solution, as the concentration of 50Mm/L. A part of this glycated protein solution was affected by unsaturated nonessential fatty acids, as the concentration of 0.5% W/V. After filtration of all samples under the sterilized condition, they were maintained in an incubator at 37°c for 16 weeks. Every 2 weeks throughout 16 weeks, an aliquot of each of solutions were prepared and then saved at -80°C until could be analyzed by fluorometry, CD (Circular Dichroism) methods, and activity assay.
Fluorometry
In this method, each of above samples at a concentration of 0.5 mg/ml was measured by Shimadzu Spectro fluorometer RF-5000 (Japan, Kyoto). Excitation and emission wavelengths of 350 and 440nm, respectively were considered.
CD (Circular Dichroism)
Spectra assessment was done by JASCO-810 spectropolarimeter (Jasco, Tokyo, Japan). The structure of each of above samples containing a concentration of 0.1 mg/ml of protein was measured. The spectra were modulated and achieved as units of mean residue molar ellipticity, [θ] (deg cm² dmol-1), based on the average weight of the amino acids (112.4). The equation [θ] λ= (θ×112.4)/cl showed the molar ellipticity and calculations were done at 25°C.
Activity
The function of each of the enzymes was measured by activity assay kits by the enzymatic colorimetric method, BiocoreDiagnosik Ulm GmbH, Germany for catalase and GPx, Biovision USA for SOD. The measurement of enzyme activity was performed as U/ml, but the results have been presented as a percentage.
Results
Statistical analysis
Box-Cox regression was used to assess the effect of adding glucose to enzymes and the effect of adding fatty acid to enzyme+glucose. Statistical significance was defined as a p-value less than 0.05.
Fluorescence spectroscopy
Changes in fluorescence emission of antioxidant enzymes including catalase, Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPX) were studied alone, in hyperglycemic condition and finally after adding each of the fatty acids. Results showed that all three enzymes had asignificant increase in fluorescence emission after incubation with glucose over time compared to the baseline fluorescence of the enzyme (p=0.000) (Table 1, Figure 1).
Enzyme+Glucose
Fluorescence
P value
CD
P value
Activity
P value
Catalase+ Glucose
0.001
0.001
0.024
SOD +Glucose
0.001
0.035
0.04
GPX+Glucose
0.001
NS
0.04
Table 1: Statistical significance of changes in fluorescence, Circular Dichroism (CD) and activity of Catalase, Superoxide Dismutase (SOD) and glutathione peroxidase after incubation with glucose.
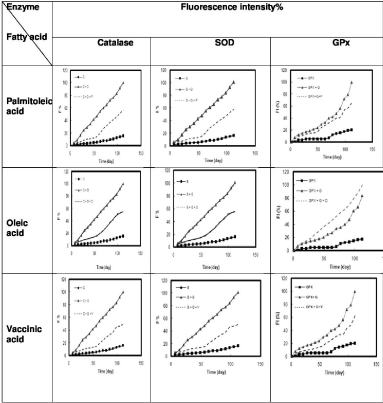
Figure 1: Percentage of Fluorescence intensity (F%) of each enzyme (Catalase, Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx)) alone,
after incubation with glucose and after incubation with glucose and each fatty acids (palmitoleic acid, oleic acid, vaccinic acid) separately over time (120 days).
The excitation and emission wavelengths were 350nm and 440nm, respectively. Right angle line: enzyme alone, Triangle line: enzyme+glucose, Dash line:
enzyme+glucose+fatty acid. P: Palmitoleic acid, O: Oleic acid, V: Vaccinic acid, C: Catalase, SOD: S, G: Glucose.
After incubation of catalase with glucose and each of the three studied fatty acids separately including palmitoleic, oleic and vaccenic acid fluorescence emission had a significant trend toward normal (palmitoleic acid p=0.005, oleic acid p=0.007, and vaccenic acid p=0.0030).
After incubation of Superoxide Dismutase (SOD) in the same conditions, fluorescence emission similarly has a significant trend toward normal status (palmitoleic acid p=0.005, oleic acid p=0.007, and vaccenic acid p=0.003).
After incubation of Glutathione Peroxidase (GPX) in the same conditions, there was not a significant change in fluorescence emission of this enzyme. (palmitoleic acid p=0.26, oleic acid p=0.14 and vaccenic acid p=0.054). There was a trend toward normal with vaccenic acid but it was not significant (Table 2, Figure 1).
Enzyme + Glucose+fatty acid
Fluorescence
P value
CD
P value
Activity
P value
Catalase+Glucose+ palmitoleic acid
0.005
NS
NS
Catalase+Glucose+ oleic acid
0.007
0.004
NS
Catalase+Glucose+ vaccinic acid
0.003
0.001
NS
SOD+ Glucose+ palmitoleic acid
0.005
0.001
NS
SOD+Glucose+Oleic acid
0.007
0.001
NS
SOD+Glucose+Vaccinic acid
0.003
0.001
NS
GPx+Glucose+Palmitoleic acid
NS
NS
NS
GPx+Glucose+Oleic acid
NS
NS
NS
GPx+Glucose+Vaccenic acid
NS
NS
NS
Table 2: Statistical significance of changes in fluorescence, circular dichroism and activity of each enzyme (Catalase, SOD and GPX) after incubation with glucose and each fatty acid separately (palmitoleic, oleic and vaccinic acid) compared to incubation with glucose alone.
CD spectra
Changes in the secondary structure of antioxidant enzymes including catalase, Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPX) were investigated with Circular Dichroism (CD), alone, in hyperglycemic condition and finally after adding each of the fatty acids.
After incubation of catalase and SOD with glucose over time there were significant changes in CD compared to CD of each two enzymes alone. (catalase p=0.000, SOD p=0.035) But there was not a significant change in CD of glutathione peroxidase after incubation with glucose over time in comparison to CD of this enzyme alone (p=0.52) (Table 1).
After incubation of catalase with both glucose and palmitoleic acid, CD had no significant trend toward normal (p=0.092), but after incubation of this enzyme with both glucose and oleic acid or glucose and vaccenic acid, there was asignificant change in CD toward normal (p=0.004 and p<0.001).
After incubation of Superoxide Dismutase (SOD) with glucose and each of the studied fatty acids, CD showed a significant trend toward to normal (p<0.001).
After incubation of Glutathione Peroxidase (GPX) in the same conditions, therewas no significant change in CD of this enzyme (palmitoleic acid p=0.68, oleic acid p=0.79, vaccenic acid p=0.51) (Table 2, Figure 2).
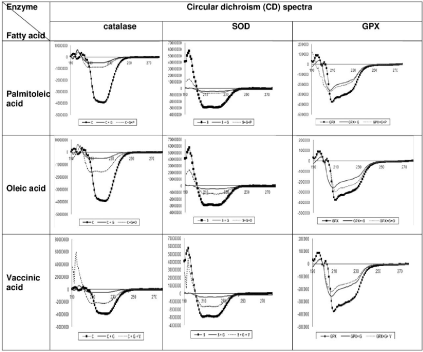
Figure 2: changes in Circular Dichroism (CD) of each enzyme (Catalase, Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx)) alone, after incubation
with glucose and after incubation with glucose and each fatty acids separately (palmitoleic acid, oleic acid, vaccinic acid). Right angel line: enzyme alone, simple
line: enzyme+glucose, dash line: enzyme+glucose+fatty acids. P: Palmitoleic acid, O: Oleic acid, V: Vaccinic acid, C: Catalase, S: SOD, G: Glucose.
Activity
There was asignificant reduction in activity of all three enzymes after incubation with glucose over time compared to the activity of each enzyme alone (catalase p=0.024, SOD p=0.04 and GPx p=0.04) (Table 1).
After incubation of catalase with glucose and palmitoleic acid, oleic acid and vaccenic acid with each one separately, there was no significant change in activity of these three enzymes compared to incubation with glucose alone (palmitoleic acid p=0.73, oleic acid p=0.57 and vaccenic acid p=0.52).
After incubation of SOD with glucose and palmitoleic acid, oleic acid and vaccenic acid with each separately, there was no significant difference in SOD activity compared to incubation with glucose alone (palmitoleic acid p=0.63, oleic acid p=0.57 and vaccenic acid p=0.52).
Also after incubation of Glutathione Peroxidase (GPX) with glucose and palmitoleic acid, oleic acid and vaccenic acid with each separately, there was no significant difference in GPX activity compared to incubation with glucose alone (palmitoleic acid p=0.53, oleic acid p=0.48 and vaccenic acid p=0.49) (Table 2, Figure 3).
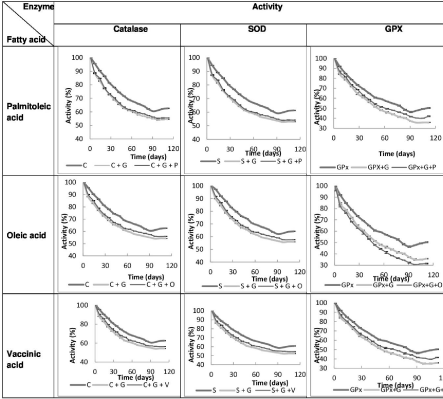
Figure 3: changes in Activity of each enzyme (Catalase, Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx)) alone, after incubation with glucose
and after incubation with glucose and each fatty acid separately (palmitoleic acid, oleic acid, vaccinic acid). P: Palmitoleic acid, O: Oleic acid, V: Vaccinic acid, C:
Catalase, S: SOD, G: Glucose.
Discussion
This study revealed that hyperglycemic environment induced significant changes in fluorescence emission and activity of catalase, SOD, and GPx. In contrast to catalase and SOD, the secondary structure of the GPx was resistant to hyperglycemic damage.
Catalase and SOD showed significant changes toward normal in fluorescence emission after incubation with glucose and each the three fatty acids compared to incubation with glucose alone, but GPx showed no significant change. It means that damage in total structure of GPx in hyperglycemic condition could not be protected with the addition of mentioned unsaturated fatty acids.
There was a significant change in CD of catalase and SOD toward normal after incubation with glucose and each fatty acids, except catalase and palmitoleic acid. In this study, we noticed protection of total and secondary structure of catalase and SOD with fatty acids against the hyperglycemic environment.
Following addition of both glucose and each of the fatty acids to each enzyme, activity had a trend toward normal, but the changes were not statistically significant. None of the fatty acids could protect these enzymes from a reduction in activity in hyperglycemic condition.
Lucchesi et al in their experimental study showed anincreased concentration of free radicals in the liver and significant reduction of antioxidant activity of the enzymes SOD, Catalase and GPx in alloxan - induced diabetic rats [21]. This is in agreement with the finding of our study.
In a study in 2002, Massaro M et al. showed quenching of ROS generation with oleate [22]. Richard et al in 2008 showed that polyunsaturated fatty acids reduced ROS production and direct superoxide scavenging [23]. In 1999, Ruiz-Gutierrez V et al. studied the effect of oleic-rich diets and fish oil on lipid composition and antioxidant enzymes in nondiabetic rats. They showed higher activities of Catalase, SOD, and GPx in fish oil fed rats compared to oleic acid rich diets [17]. We studied both structural changes and activity of these enzymes in interaction with monounsaturated fatty acids in hyperglycemic condition.
Another study in 2013 revealed flaxseed oil upregulated activity and expression of Catalase and SOD and expression of GPx in the liver of streptozocin-nicotinamide induced diabetic rats. Whereas fish oil up-regulated activity and expression of Catalase [24]. In the present study, although there was protection against structural damage in the hyperglycemic condition in Catalase and SOD, there was not a significant change in activity of these enzymes in interaction with mentioned fatty acids in hyperglycemic condition.
In our study, GPx showed different behavior compared to catalase and SOD. Changes in fluorescence emission of this enzyme in hyperglycemic condition could not be protected with the addition of the studied fatty acids.
GPx is an antioxidant enzyme, glutathione reductase and glucose 6 phosphate dehydrogenase and cofactors such as reduced glutathione and NADPH are needed for its action [8]. It is a selenoprotein enzyme that can metabolize hydrogen peroxide and lipid hydroperoxides [25]. Lipid peroxidation is a process that oxidants attack lipid containing carbon- carbon double bonds especially polyunsaturated fatty acids. The main primary products of lipid peroxidation are lipid hydroperoxides (LOOH) [26]. GPx may be effective in detoxifying fatty acid hydroperoxides [27]. Our study represents a structural damage and reduction in activity of antioxidative enzymes in high glucose concentration. The decrease in antioxidant capacity could be related to the diabetes complications [28]. The effect of nonessential unsaturated fatty acids on the structure of these antioxidant enzymes may help to the prevention of diabetes complications.
Conclusion
This study provides preliminary evidence for the preventive role of nonessential unsaturated fatty acids against damage of high glucose concentration on the structure of anti- oxidative enzymes including catalase and SOD but not in GPx. Elucidating implications of these findings in relation to diabetic microvascular and macrovascular complications can be a focus for future research.
References
- Fujita H, Fujishima H, Chida S, Takahashi K, Qi Z, Kanetsuna Y, et al. Reduction of renal superoxide dismutase in progressive diabetic nephropathy. Clin J Am Soc Nephrol. 2009; 20: 1303-1313.
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985; 227: 375- 381.
- Faraci FM, Didion SP. Vascular protection superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004; 24: 1367-1373.
- Goth L, Eaton JW. Hereditary catalase deficiencies and increased risk of diabetes. Lancet. 2000; 356: 1820-1821.
- Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes. 1998; 47: 1578- 1585.
- Mirault M, Tremblay A, Beaudoin N, Tremblay M. Overexpression of selenoglutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem. 1991; 266: 20752- 20760.
- Kelner MJ, Bagnell RD, Uglik SF, Montoya MA, Mullenbach GT. Heterologous expression of selenium-dependent glutathione peroxidase affords cellular resistance to paraquat. Arch Biochem Biophys. 1995; 323: 40-46.
- De Haan JB, Bladier C, Griffiths P, Kelner M, O’Shea RD, Cheung NS, et al. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stressinducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998; 273: 22528-22536.
- Bakala H, Hamelin M, Mary J, Borot-Laloi C, Friguet B. Catalase, a target of glycation damage in rat liver mitochondria with aging. Biochim Biophys Acta. 2012; 1822: 1527-1534.
- Suravajjala S, Cohenford M, Frost LR, Pampati PK, Dain JA. Glycation of human erythrocyte glutathione peroxidase: Effect on the physical and kinetic properties. Clin Chim Acta. 2013; 421: 170-176.
- Anwar S, Khan MA, Sadaf A, Younus HA. Structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int J Biol Macromol. 2014; 69: 476-481.
- Bagnyukova TV, Vasylkiv OY, Storey KB, Lushchak VI. Catalase inhibition by amino triazole induces oxidative stress in goldfish brain. Brain res. 2005; 1052: 180-186.
- Kravchenko L, Aksenov I, Avren’eva L, Beketova N, Trusov N, Guseva G. Effects of omega-3 polyunsaturated fatty acids on antioxidant capacity in rats. Vopr Pitan. 2013; 82: 4-9.
- Hunina L, Chekman I, Nebesna T, Horchakova N. Effectiveness of use of omega-3 polyunsaturated fatty acids at physical loads. Fiziol Zh. 2013; 59: 68-77.
- Muldoon MF, Erickson KI, Goodpaster BH, Jakicic JM, Conklin SM, Sekikawa A, et al. Concurrent physical activity modifies the association between n3 long-chain fatty acids and cardiometabolic risk in midlife adults. J Nutr. 2013; 143: 1414-1420.
- Kruse L, Ogletree Jr R. Omega-3 fatty acids and cardiovascular risk. J Miss State Med Assoc. 2013; 54: 156-157.
- Ruiz-Gutierrez V, Perez-Espinosa A, Vázquez CM, Santa-Maria C. Effects of dietary fats (fish, olive and high-oleic-acid sunflower oils) on lipid composition and antioxidant enzymes in rat liver. Br J Nutr. 2013; 82: 233-241.
- Rifai N, Warnick GR. Lipids, lipoproteins, apolipoproteins, and other cardiovascular risk factors. Tietz textbook of clinical chemistry and molecular diagnostics. 2006; 26: 903-981.
- Qian F, Korat AA, Malik V, Hu FB. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2016; 39: 1448- 1457.
- Alcubierre N, Navarrete-Muñoz EM, Rubinat E, Falguera M, Valls J, Traveset A, et al. Association of low oleic acid intake with diabetic retinopathy in type 2 diabetic patients: a case-control study. Nutr Metab. 2016; 13: 40.
- Lucchesi AN, Freitas NTd, Cassettari LL, Marques SFG, Spadella CT. Diabetes mellitus triggers oxidative stress in the liver of alloxan-treated rats: a mechanism for diabetic chronic liver disease. Acta Cir Bras. 2013; 28: 502- 508.
- Massaro M, Basta G, Lazzerini G, Carluccio MA, Bosetti F, Solaini G, et al. Quenching of intracellular ROS generation as a mechanism for oleateinduced reduction of endothelial activation and early atherogenesis. Thromb Haemost. 2002; 88: 335-344.
- Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008; 57: 451-455.
- Jangale NM, Devarshi PP, Dubal AA, Ghule AE, Koppikar SJ, Bodhankar SL, et al. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chem. 2013; 141: 187-195.
- Arthur J. The glutathione peroxidases. Cell Mol Life Sci. 2001; 57: 1825-1835.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative medicine and cellular longevity. 2014.
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998; 39: 1529-1542.
- Lodovici M, Giovannelli L, Pitozzi V, Bigagli E, Bardini G, Rotella CM. Oxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic control. Mutat Res. 2008; 638: 98-102.
Citation: Mirmiranpour H, Rabizadeh S, Mansournia MA, Salehi SS, Esteghamati A and Nakhjavani M. Protective Effect of Palmitoleic, Oleic, and Vaccenic Acid on Structure -Function of Major Antioxidant Enzymes: Catalase, Superoxide Dismutase and Glutathione Peroxidase in the Hyperglycemic Environment: an In Vitro Study. Austin Biochem. 2018; 3(1): 1017.