
Short Communication
Austin Biochem. 2019; 4(1): 1020.
On Formation of Ephippium in Wlassicsia Pannonica Daday, 1904 (Crustacea, Cladocera)
Makrushin A.V1*, Petrosyan A.G2, Dyatlov S. Ye2, Koshelev V.A2, A.V. Makrushin1
1Institute for Biology of Inland Waters, Borok Yaroslavl Region, Russia
2Odessa Branch of the Institute of Biology of Southern Seas, Odessa, Ukraine
*Corresponding author: Makrushin VA, Institute for Biology of Inland Waters, Borok Yaroslavl Region, Russia
Received: December 28, 2018; Accepted: January 08, 2019; Published: January 15, 2019
Abstract
During transition to gamogenesis in females of Wlassicsia pannonica an outer layer of shell chitin thickens slightly and the inner layer becomes much thicker locally and acquires similarity with honeycomb-like chitin covering outer part of shells of ephippium in Daphniidae and Moinidae. In interpretation of [1] Macrothricinae is a polyphyletic group as the process of ephippium formation in its representatives differs significantly.
Keywords: Wlassicsia pannonica; Crustacea; Cladocera; Macrothricinae ephippium
Introduction
Histological studies of cladocerans allow finding details of ephippium formation that are impossible to be uncovered using other methods, specify the idea on species relation degree and enhance the taxonomy of these crustaceans. Initial form of ephippium was shell’s leaflets chitin which does not differ from chitin of parthenogenetic females. Such ephippium is still present in Bosminidae [2] and in Ophryoxus gracilis [3]. Chitin formed by the outer leaf has changed comparing with the initial one in Daphniidae [4-6], Moinidae and Bunops serricaudata [3]. Chitin formed by the inner leaf has changed in Streblocerus serricaudatus and Drepanotrix dentata (Macrothricidae) [3]. Hypoderma of other body parts takes part in ephippium formation in some species. Chitin covering dorsal side of the body became part of the ephippium in Chydoridae [7,8], while in Lathonura rectirostris ephippium is formed by the chitin of rectum and post abdomen [9,10]. Sticky mucus serving the purpose of attaching ephippium to underwater objects is discharged by the inner leaf of the shell’s hypoderma under the chitin inlaying the brood chamber from the inside [11,12] in Acantholeberis curvirostris. Aim of the present study was to examine the formation of ephippium in Wlassicsia pannonica (Macrothricidae).
Material and Methods
W. pannonoca were found in the splash zone of Dofinovsky estuary of the Black Sea and bred in the laboratory. They were fed with commercial baker’s yeast and Chlorella. Fixation was performed using Bouin’s fluid. Paraffin sections (7μ) were stained in haemotoxyline according to Heidenhain.
Results and Discussion
Transversal sections of following types of females are given on figures: parthenogenetic (Figure 1A), in transition from parthenogenesis to gamogenesis (Figure 1B) and two gamogenetic ones (C,D). Eggs are still in the ovaries in one of the gamogenetic females (Figure 1(C5)) and in the brood pouch in the other (Figure 1(D2)). Stages of ephippium formation are shown on (Figures 1(B→C→D)). It is seen that outer chitin has exfoliated (Figure 1(B3, C3, D3)). It is significantly thicker than in the parthenogenetic female and S. serricaudatus are as big as they are between Daphniidae and Chydoridae, for instance. Macrothricinae as it is thought of by Dumont and Silva-Briano include representatives of at least three long diverged phylogenetic lines: first-Wlassicsia, second-Streblocerus and Drepanotrix, third-Bunops. The ephippium is similar in Streblocerus and Drepanotrix [3]. The system of Macrothricinae by [1] is based upon the results of studies of parthenogenetic females. However, gamogenetic females possess features important from the taxonomic point of view, absent in parthenogenetic females and they should be considered.
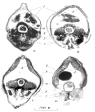
Figure 1: Transverse sections of Wlassicsia pannonica, demonstrating
formation of ephippium in this species. A-parthenogenetic female, B-female
turning from parthenogenesis to gamogenesis, C, D-gamogenetic females;
1- parthenogenetic embryos in a brood pouch, 2-latent egg in a brood pouch,
3-amorphic outer chitin exfoliated from the hypoderma, 4-structured chitin
lining an inside part of a brood pouch, 5-ovaries, 6-gut, 7-legs.
References
- Dumont HJ, Silva-Briano M. A reclassification of the anomopod families Macrothricidae and Chydoridae, with the creation of a new suborder, the Radopoda (Crustacea: Branchiopoda). Hydrobiologia. 1998; 384: 119-149.
- Makrushin AV. Resistance to drying of latent eggs of Bosmina obtusirostris (Cladocera, Crustacea) and their reactivation. Zoologichesky Zhurnal. 1989; 68: 132-134.
- Makrushin AV, Markevich GI. On formation of ephippium in some Cladocera (Crustacea). Zoologichesky Zhurnal. 1982; 61: 1425-1428.
- Wolff M. Studies on cuticular genesis and structure and their relationship to the physiology of the matrix. Biol Centralbl Bd. 1904; 24: S644-650, 697-722, 761-767.
- Zwack A. The finer construction and formation of the Ephippium of Daphnia hyalina Lyidig. Z wiss Zool. 1905; 79: S548-S573.
- Zwack A. Das Ephippium von Simocephalus vetulus Schoedler. Z wiss Zool Bd. 1907; 86: S304-S310.
- Makrushin AV. On structure of ephippium of Eurycercus lamellatus. Biologia vnutrennih vod. Informacionny bulleten. 1967; 27-31.
- Makrushin AV. Changes in the organisms of females of some species of Cladocera (Crustacea) under their transition to gamogenesis. Zoologichesky Zhurnal. 1970; 49: 1573-1575.
- Makrushin AV. Protoephippial glands in Cladocera (Crustacea). Zoologichesky Zhurnal. 1972; 51: 1736-1738.
- Fryer G. Observations on the ephippia of certain macrothricid cladocerans. Zool J Linn Soc. 1972; 51: 79-96.
- Makrushin AV. Some patterns of the reproductive system in Cladocera (Crustacea). Zoologichesky Zhurnal. 1976; 55; 1143-1148.
- Makrushin AV. The diversity in the structure of ephippium in the Macrothricidae (Crustacea, Cladocera) and the taxonomic status of this family. Zoologichesky Zhurnal. 1985; 64: 212-216.