Abstract
In manure, the endocrine disruptors may have a dual source: as natural metabolites of the steroidal hormones present in the manure deposed by farm animals or of synthetic origin as the alkylphenols used for a variety of applications and consumer products. In particular, the EDC with estrogen-like activity cause a number of issues mainly related to the male fertility. This study aims to ascertain if an estrogenic environment is able to induce male gonad of the lizard Podarcis sicula to the synthesize Vitellogenin (VTG), the main biomarker of estrogenic exposure. From our investigations of in situ hybridization and immunohistochemistry emerges that VTG gene is transcribed and translated in the testis of lizards collected in organic farming where the manure is the unique fertilizer used and in the samples fed with food experimentally polluted by an estrogen-like substance i.e. nonylphenol. The same findings are recorded after the injection of estradiol-17β.
Keywords: Manure; Nonylphenol; Endocrine disruptor chemicals; Testis; Vitellogenin; Lizard
Abbreviations
E2: Estradiol 17-β; NP: Nonylphenol; EDC: Endocrine Disruptor Chemical; VTG: Vitellogenin; ISH: In Situ Hybridization; IHC: Immunohistochemistry
Introduction
Many substances in the environment mimic the endogenous hormones and interfere with the endocrine system [1] in particular with the males reproductive physiology [2,3]. These substances also known as Endocrine Disruptor Chemicals (EDC), originate from a variety of sources. They may be present in the co-formulants of pesticide as the alkylphenol polyethoxylates or, in form of metabolites of steroid hormones in the manure used for fertilization of the soils dedicated to organic farming [1,4,5].
The most commercially important alkylphenol is the Nonylphenol (NP), persistent and bioaccumulable, used primarily to produce surfactants for a wide variety of applications and consumer products [1]. Due to its structural similarity with estradiol-17β, the NP is able to bind to the estrogen receptors and to stimulate the transcription of the downstream genes [6] as the estrogen receptors itself and Vitellogenin (VTG).
Little attention is given to the metabolites of steroid hormones in manure. Farm animals excrete conjugated steroid hormones that persist in the manure for several months [7]. The main problem is that the conjugated and biologically inactive forms of the hormones are easily converted into free steroids by soil microorganisms as Escherichia coli [8-10].
Many biomarkers and bioindicator species have been identified to use in screening programs to determine the presence of the EDC in the environment. The most validated biomarker of estrogenic exposure is the induction in males of Vitellogenin (VTG) the major egg yolk precursor in oviparous and ovoviviparous females [11,12]. Generally, VTG gene is expressed in the liver of mature females and is silent in males where it may be activated by estrogenic exposure at non physiological level. Most of the research in this field are conducted on aquatic or semiaquatic organisms and are focused on the hepatic induction of VTG in males living in polluted environments [13-15]. In the terrestrial experimental model such as the lizard Podarcis sicula, the E2 administration elicits (determines) the hepatic expression of VTG and ERα [24].
To date, the possible extrahepatic expression of VTG has received little or no attention [16]. In particular VTG was found in the testis of Torpedo marmorata, Melanotaenia fluviatilis and Tanichthys albonube [17-19] exposed to estrogenic chemicals. Our recent observations suggested that the treatment with estradiol-17β elicits the transcription and translation of VTG in the testis of Podarcis too [20].
So, the aim of the present research was to further investigate the possible induction of VTG in the lizard testis in two different experimental conditions. For this purpose In Situ Hybridization (ISH) and Immunohistochemistry (IHC) were used to detect the presence of VTG mRNA and protein in Podarcis samples collected in organic farming where the manure is the only fertilizer used or in samples fed with food polluted by NP. The results were compared with those obtained in the lizards injected with estradiol-17β.
Materials and Methods
Animals and experimental treatment
Sexually mature males Podarcis sicula (7-8 cm snout-vent length) were caught during their mating period (May-June) in two organic farms both located in Sorrento Peninsula (Campania, Italy): 10 animals were from a site near Gragnano (BIO-A) and 10 from a site near Agerola (BIO-B group) (Figure 1). The two sites are certified as exclusively organic farms by the Italian Department of Agriculture. Both farms are large (approx. 800 m2), perched on a hill, far away and isolated from non-organic crops. These sites use the manure of animals bred in the same farms as fertilizer.
Figure 1: Map showing the location of the two different organic agricultural
sampling site: a), BIO-A site, Sant’Antonio Abate, (Campania, Italy); b) BIO-B
site, Agerola, (Campania, Italy).
Further, thirty lizards were caught on the outskirts of Naples in an uncultivated with no previous signs of estrogenic contamination [21-23]. These wild caught animals were randomly divided into three groups. First group of ten lizards undergoing treatment with NP (Etravon-Syngenta, Italy), was fed every other day for 2 weeks with larvae of Tenebrio molitor sprayed with an aqueous NP solution (0.25%); a drinking trough containing water polluted with NP (0.05%) was always available. The live mealworms were sprayed with NP outside the terraria and then transferred inside. Their ingestion by the lizards, which eat only living and mobile larvae, was observed. Second group of ten samples were experimentally treated intraperitoneally with 17β-estradiol (168 ng/100 ml) in reptile physiological solution (NaCl 0.07%) every other day for 2 weeks [24] as control of the estrogen response. Third group of ten untreated wildlife animals, were considered as a control of the reproductive stage.
All the animals were killed by decapitation after anesthesia in ice and the testes were immediately excised and processed for ISH and IHC investigations. We were authorized to capture the animals for experimental treatments by the Italian Ministry of the Environment (auth. SCN/2D/2000/9213) and the experiments were carried out in compliance with the ethical provisions enforced by the European Union and authorized by the National Committee of the Italian Ministry of Health on in vivo experimentation (Department for Veterinary Public Health, Nutrition and Food Safety).
In situ hybridization
The testis were fixed for about 6 h in Bouin’s solution and then dehydrated in graded ethanol and embedded in paraffin wax. ISH was performed on adjacent sections with VTG cDNA probe, as previously described [21,23,25]. Briefly, the sections dewaxed in Xilene solution and rehydrated in graded ethanol, were treated with proteinase K (10 lg/ml) at 50°C for 10 min. Digoxigenin (DIG)-labeled probes were used at a concentration of 80 ng/100μl in hybridization buffer (Tris-HCl 0.02 M, pH 7.5; NaCl 0.3 M; EDTA 0.01 M; DTT 0.1 M; Formamide 50%; Denhardt’s 1x; tRNA 100 μg/ml; ss-DNA 100 μg/ ml) overnight at 50°C in a moist chamber. The slides were incubated with RNase mix at 37°C for 30 min and in the same mix without RNase at 37°C for 30 min, washed in 2x SSC for 3 min, in 0.1x SSC at 60°C 15 min, and in NTP (Tris-HCl 0.1 M, pH 7.5; NaCl 0.15 M) and then incubated in 2% blocking solution (Roche Diagnostics, Mannheim, Germany) in maleic acid buffer (0.1 M maleic acid; 0.15 M NaCl, pH 7.5) for 1 h. The sections were kept overnight at 4°C with an alkaline phosphatase-conjugated sheep anti-DIG antibody (Roche Diagnostics) (1:2500) in blocking solution and rinsed in NTP buffer for 30 min and in NTM buffer (Tris-HCl 100 mM, pH 9.5; MgCl 50 mM; NaCl 100 mM) for 30 min. Finally, the sections were kept in the color detection substrate solution BCIP/NBT (nitro-blue tetrazolium and 5-bromo-4-chloro-30-indolyphosphate) in the dark at room temperature as recommended by the manufacturer (Roche) in NTM until appearance of the color. For negative control, the hybridization solution did not contain cDNA probes.
Immunohistochemistry
For VTG localization, testis sections were incubated with an homologous primary anti-VTG antibody (1:1000) [26] in phosphate buffer (PB) 0.1 M pH 7.4 overnight at 4°C, washed in the same buffer and incubated with secondary polyclonal biotinylated antirabbit antibody (Pierce, Rockford, USA) (1:500 in PB). To detect antigen, ultrasensitive ABC staining reagent kit (Pierce, USA) and diaminobenzidine (DAB 1 mg/ml; Sigma) was used. Negative control sections were obtained by omitting incubation with primary antibody.
Results
In all the samples captured in BIO areas (Figure 2A,B) or exposed to the NP-polluted diet (Figure 2C,D) VTG-mRNA (Figure 2A,C) or VTG protein (Figure 2B,D) were detected in all cells of the seminiferous epithelium. In the animals injected with E2, showing a reduction of the seminiferous epithelium as the NP samples, the same positivity to ISH (Figure 2E) and IHC (Figure 2F) was recorded.
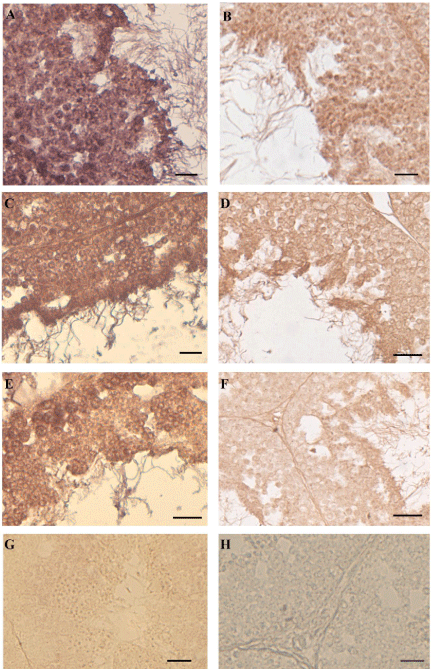
Figure 2: In situ hybridization and immunocytochemistry in the testis of
Podarcis. To the left of the panel in situ hybridization with VTG probe: in
BIO (A), NP-treated (C) and E2-treated (E) samples all germ cells of the
seminiferous epithelium express VTG-mRNA. No VTG-mRNA is detected in
wildlife untreated males (G). To the right of the panel immunohistochemistry
with anti-VTG antibody: in BIO (B), NP-treated (D) and E2-treated (F) all
germ cells synthesize VTG-protein. No positivity was evident in the control
reactions (one for all is depicted in H). The bar is 30 μm.
In the testis of untreated wild males no VTG-mRNA neither VTG protein was detected (one for all is depicted in Figure 2G). The sections incubated without VTG probe or without primary antibody as a control of reactions displayed always negative results (one for all is depicted in Figure 2H).
Discussion
The present results evidencing the expression of VTG in the testis of lizards living in the areas of organic farming where the manure is the unique fertilizer used, suggest that the manure may have estrogenic action as NP and E2.
Therefore, being the testis the site of germ cells production, this anomalous synthesis of VTG may threat the fertility and then the continuity of the specie.
Podarcis mature females physiologically synthesize VTG during the breeding season. In this period each ovulatory wave is characterized by a peak of E2 and VTG, in non-breeding period the levels of endogenous E2 are low and plasma VTG is absent [27,28]. In wild males this protein is never detected in any tissue neither when the levels of circulating E2 are the highest for males during the postmating refractory period [29]. Nevertheless it’s widely demonstrated in the same lizard [24,25] as in other oviparous vertebrates [12,27,30- 33] that the estrogen are able to trigger the expression of VTG gene in the liver of males.
In the referred experimental plain were considered two conditions in which the wild animals may live in the environment polluted by estrogen-like substances. In the first case, the lizards were captured in the fields devoted to BIO-farming where the manure is the main used fertilizer. In the second, the potential conditions to which the terrestrial vertebrates may be exposed by eating and drinking in xeno-estrogenic polluted fields were experimentally mimicked. The results of ISH and IHC undoubtedly showed that, both in BIO animals and NP-exposed males, the testis was able to transcribe and translate VTG as in the E2 treated males. This confirms once again the xeno-estrogenic action of the manure and NP already observed by us in the liver of this terrestrial vertebrate [25,34].
The researches on the extrahepatic synthesis of VTG are really scarce and are conducted mainly in male fishes exposed to E2 or NP [35,36].
In the samples of Podarcis here analyzed, the presence of VTGmRNA in all stage of spermatogenesis proves clearly that the VTG synthesis takes place in situ and therefore the germ cells are the target of the estrogenic action of manure and NP.
Moreover, the anomalous presence of VTG in the testis may be prelude of reproductive impairment, compromising the fertility of Podarcis. It is known that in this lizard the treatment with E2 or NP induces a slowdown of spermatogenesis with consequent alteration of the testicular structure [20,37]. Also in the samples here examined it is evident that E2 or NP during the reproductive period determines a reduction of the seminiferous epithelium affecting the testicular structure making it similar to that of the non-reproductive period. Further in rat, NP induces impairment of sperm function and motility [38].
Lizards are important components of the food webs in most ecosystems. They fill a critical role both as predator and prey species: they are an important prey for birds, snakes and other animals, and are important predators of insects. Podarcis can be directly exposed through various routes, including: ingestion of contaminated food and/or soil, inhalation, dermal exposure. So, Podarcis appears to be very useful terrestrial non-mammalian bio-indicator specie and VTG, the rapid inducible biomarker, is once again a valuable assay to easily verify the damages caused by EDC.
In synthesis, taken together these results open a number of issues on the use, both in BIO-farming and in the traditional agriculture, of fertilizers which can cause alteration of the endocrine homeostasis. All this, besides to represent a serious problem for the food chain, poses a serious question for the continuity of this species as of the other animal species. Their disappearance in fact, could have an ecological importance greater than what one might think since the removal of any species from its ecosystem can drastically alter the populations of other organisms.
Conclusion
In conclusion, these results demonstrated for the first in a terrestrial vertebrate the ability of the testis to synthesize VTG. In males, the activation of an estrogen-dependent gene i.e. VTG, in the two reported experimental condition, may be due to the presence of estrogen metabolites in manure used to fertilize the fields on which the lizards were collected for this study or to diet experimentally polluted by an estrogen-like substance as NP.
Acknowledgement
The author would like to express her deepest gratitude to Prof. Ermelinda Liamtola for her valuable critical review of this manuscript.
References
- Priac A, Morin-Crini N, Druart C, Gavoille S, Bradu C, Lagarrigue C, et al. Alkylphenol and alkylphenol polyethoxylates in water and wastewater: A review of options for their elimination. Arabian Journal of Chemistry 2014.
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010; 365: 1517-1535.
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011; 84: 207-217.
- Bartelt-Hunt SL, Devivo S, Johnson L, Snow DD, Kranz WL, Mader TL, et al. Effect of composting on the fate of steroids in beef cattle manure. J Environ Qual. 2013; 42: 1159-1166.
- Valdehita A, Quesada-Garcia A, Delgado MM, Martin JV, Garcia-Gonzalez MC, Fernandez-Cruz ML, et al. In vitro assessment of thyroidal and estrogenic activities in poultry and broiler manure. Sci Total Environ. 2014; 472: 630-641.
- Celius T, Haugen TB, Grotmol T, Walther BT. A sensitive zonagenetic assay for rapid in vitro assessment of estrogenic potency of xenobiotics and mycotoxins. Environmental Health Perspectives. 1999; 107: 63-68.
- Schiffer B, Daxenberger A, Meyer K, Meyer HH. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: environmental studies. Environ Health Perspect. 2001; 109: 1145-1151.
- Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent: Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998; 32: 1549-1558.
- Ternes TA, Kreckel P, Mueller J. Behaviour and occurrence of estrogens in municipal sewage treatment plants-II. Aerobic batch experiments with activated sludge. Sci Total Environ. 1999; 225: 91-99.
- Baronti C, Curini R, D’Ascenzo G, Di Corcia A, Gentili A, Samperi R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol. 2000; 34: 5059-5066.
- Sumpter JP, Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995; 103: 173-178.
- Wallace RA. Vitellogenesis and oocyte growth in nonmammalian vertebrates. Dev Biol (N Y 1985). 1985; 1: 127-177.
- Ahel M, McEvoy J, Giger W. Bioaccumulation of the lipophilic metabolites of nonionic surfactants in freshwater organisms. Environ Pollut. 1993; 79: 243-248.
- Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem. 1996; 15: 194-202.
- El-Sayed Ali T, Abdel-Aziz SH, El-Sayed AF, Zeid S. Structural and functional effects of early exposure to 4-nonylphenol on gonadal development of Nile tilapia (Oreochromis niloticus): a-histological alterations in ovaries. Fish Physiol Biochem. 2014; 40: 1509-1519.
- Shanthanagouda AH, Nugegoda D, Hassell KL, Patil JG. Exposure to estrogenic chemicals induces ectopic expression of VTG in the testis of rainbowfish, Melanotaenia fluviatilis. Bull Environ Contam Toxicol. 2013; 91: 438-443.
- Del Giudice G, Prisco M, Agnese M, Verderame M, Rosati L, Limatola E, et al. Effects of nonylphenol on vitellogenin synthesis in adult males of the spotted ray Torpedo marmorata. J Fish Biol. 2012; 80: 2112-2121.
- Shanthanagouda AH, Nugegoda D, Hassell KL, Patil JG. Exposure to estrogenic chemicals induces ectopic expression of vtg in the testis of rainbowfish, Melanotaenia fluviatilis. Bull Environ Contam Toxicol. 2013; 91: 438-443.
- Wang RL, Gao Y, Zhang LH, Zhang YK, Fang ZQ, He JG, et al. Cloning, expression, and induction by 17-beta estradiol (E-2) of a vitellogenin gene in the white cloud mountain minnow Tanichthys albonubes. Fish Physiol Biochem. 2010; 36: 157-164.
- Verderame M, Limatola E, Scudiero R. Ectopic synthesis of vitellogenin in testis and epididymis of estrogen-treated lizard Podarcis sicula. General and Comparative Endocrinology. 2016.
- Verderame M, Angelini F, Limatola E. Expression of estrogen receptor alpha switches off secretory activity in the epididymal channel of the lizard Podarcis sicula. Mol Reprod Dev. 2012; 79: 107-117.
- Verderame M, Angelini F, Limatola E. Research Article Spermatogenic Waves and Expression of AR and ERs in Germ Cells of Podarcis sicula. International Journal of zoology. 2014.
- Verderame M. The involvement of the androgen receptor in the secretion of the epididymal corpus in the lizard Podarcis sicula. Int J Zool. 2014.
- Verderame M, Limatola E. Molecular identification of estrogen receptors (ERα and ERβ) and their differential expression during VTG synthesis in the liver of lizard Podarcis sicula. Gen Comp Endocrinol. 2010; 168: 231-238.
- Verderame M, Prisco M, Andreuccetti P, Aniello F, Limatola E. Experimentally nonylphenol-polluted diet induces the expression of silent genes VTG and ERα in the liver of male lizard Podarcis sicula. Environ Pollut. 2011; 159: 1101-1107.
- Rosanova P, Romano M, Marciano R, Anteo C, Limatola E. Vitellogenin precursors in the liver of the oviparous lizard, Podarcis sicula. Mol Reprod Dev. 2002; 63: 349-354.
- Carnevali O, Mosconi G, Angelini F, Limatola E, Ciarcia G, Polzonetti-Magni A. Plasma vitellogenin and 17 beta-estradiol levels during the annual reproductive cycle of Podarcis sicula Raf. Gen Comp Endocrinol. 1991; 84: 337-343.
- Verderame M, Cafiero G, Limatola E. Vitellogenin, oestradiol 17-β and its receptors in the lizard Podarcis sicula: cross-talk between growing oocytes and liver. In: Advances in Zoology Research, NOVA Publisher. 2012; 2: 245-258.
- Angelini F, Botte V. Spermatogenesis in reptiles: dynamic and regulatory aspects. Dallai R, editor. In: Sex Origin and Evolution. Mucchi Selected Symposia and Monographs UZI, Modena, Italy. 1992; 211-230.
- Whali W. Evolution and expression of vitellogenina genes. Trends in Genetics. 1988; 4: 227-232.
- Carnevali O, Sabbieti MG, Mosconi G, Polzonetti-Magni AM. Multihormonal control of vitellogenin mRNA expression in the liver of frog, Rana esculenta. Mol Cell Endocrinol. 1995; 114: 19-25.
- Cerda J, Bruce BG, LaFleur GJ Jr, Limesand S. Pattern of vitellogenin and follicle maturational competence during the ovarian follicular cycle of Fundulus Heteroclitus. General and Comparative Endocrinology. 1996; 103: 24-35.
- Kobayashi K, Tamotsu S, Yasuda K, Oishi T. Vitellogenin-immunohistochemistry in the liver and the testis of the Medaka, Oryzias latipes, exposed to 17bestradiol and p-nonylphenol. Zoological Science. 2005; 22: 453-461.
- Verderame M, Limatola E, Scudiero R. Estrogenic contamination by manure fertilizer in organic farming: a case study with the lizard Podarcis sicula. Ecotoxicology. 2016; 25: 105-114.
- Wang H, Tan JT, Emelyanov A, Korzh V, Gong Z. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene. 2005; 356: 91-100.
- Del Giudice G, Prisco M, Agnese M, Verderame M, Limatola E, Andreuccetti P. Expression of vitellogenin in the testis and kidney of the spotted ray Torpedo marmorata exposed to 17β-estradiol. Gen Comp Endocrinol. 2011; 174: 318-325.
- Verderame M, Limatola E. Interferences of an environmental pollutant with estrogen-like action in the male reproductive system of the terrestrial vertebrate Podarcis sicula. Gen Comp Endocrinol. 2015; 213: 9-15.
- Uguz C, Varisli O, Agca C, Agca Y. Effects of nonylphenol on motility and subcellular elements of epididymal rat sperm. Reprod Toxicol. 2009; 28: 542-549.
