Abstract
Solid Pseudopapillary Tumor of the Pancreas (SPTP) is one of the rare primary tumors of the pancreas. The definitive pre-operative cytological specimens that were obtained by Endoscopic Ultrasound-Guided Fine-Needle Aspiration (EUSFNA) can guide the surgical approach. The present study described a case of SPTP that underwent successful surgery after being diagnosed by EUS-FNA. A 25-year-old woman was admitted to our hospital. A mass in the pancreatic head was incidentally detected during physical examination. Ultrasonography and multidetector CT scan of the abdomen revealed a large mass composed of solid and cystic components located on the head of the pancreas. The laboratory results were all within the normal limits but the patient was mildly anemic. The serum tumor markers including serum carcinoembryonic antigen and CA 19-9 were normal. However, CA125 was slightly higher. The patient underwent EUSFNA. The cytological smear and cell block of the sample obtained by EUS-FNA revealed hypercellular nests of ductal epithelial cells with branching papillary features and a central fibrovascular core. A diagnosis of SPTP was considered. The patient underwent pancreatic mass excision. Gross specimen showed a round solid encapsulated mass, measuring approximately four centimeters, with cystic change due to hemorrhage and necrosis. The tumor was separated from the surrounding tissue. No evidence of neoplastic vascular and perineural invasion was observed. Immunohistochemical analysis of the tumor was positive for vimentin, neuron specific enolase, Progesterone receptor, β-catenin, CD10, CD56, and focal positivity for synaptophysin. However, CD99 and CagA were negative.
Keywords: Solid Pseudopapillary Tumor of the Pancreas (SPTP); Endoscopic Ultrasound-Guided Fine-Needle Aspiration (EUS-FNA)
Introduction
SPTP predominantly occurs in young women [1], approximately in 0.17-2.7% of all non-endocrine tumors of the pancreas [2]. It was first reported by Frantz in 1959 [3]. Endoscopic Ultrasound-guided (EUS-guided) Fine-Needle Aspiration (FNA) is a minimally invasive and reliable method to diagnose SPTP before surgical excision.
Method and Result
A 25-year-old woman was admitted to our hospital (the second affiliated hospital of Soochow University, China) with a pancreatic mass that was incidentally detected by routine physical examination. Ultrasonography of the abdomen revealed a large mass composed of solid and cystic components located on the head of the pancreas. The following day, a 64-row multidetector CT scan of abdomen confirmed the findings of the abdominal ultrasonography. The round mass measured 45 mm x 45 mm x 40 mm, had a clear margin, and was located in the head of the pancreas. The mass had solid and cystic components. The solid component appeared enhanced in the arterial phase, whereas the cystic part remained unenhanced (Figure 1A). On admission, laboratory tests were conducted and the results were as follows: white blood cell count, 4.1 × 10³/μL; hemoglobin, 9.8 g/dL; platelets, 242 × 10³/μL; total bilirubin, 5.4 umol/L; aspartate aminotransferase/alanine aminotransferase, 53/17 U/L; amylase/lipase, 68/29 U/L; CEA 0.47 ng/mL, CA 19-9, 9.5 U/mL, CA 125, 124.3 U/mL. The patient underwent EUS-FNA. Endoscopic Ultrasound (EUS) revealed an approximately 4.2 cm x 3.9 cm mass in the pancreatic head. The mass had a solid and cystic consistency in (Figure 1B). EUS-FNA was performed twice using a 22-gauge needle (Echotip ProCore; Cook Endoscopy, Bloomington, IN) inserted via transgastric pathway without complications. The cytological smear and cell block of the sample revealed hypercellular nests of ductal epithelial cells with branching papillary features and a central fibrovascular core (Figure 2). Based on the characteristic histology of the sample, a diagnosis of Solid Pseudopapillary Tumor of the Pancreas (SPTP) was considered. The patient underwent pancreatic mass excision. Gross specimen showed a round solid encapsulated mass, measuring approximately four centimeters, with cystic change due to hemorrhage and necrosis (Figure 3). The tumor was separated from the surrounding tissue. No evidence vascular and perineural neoplastic invasion was observed. Immunohistochemical analysis of the sample was positive for vimentin, Neuron Specific Enolase (NSE), Progesterone Receptor (PR), β-catenin, CD10, CD56, and focal positivity for synaptophysin (syn). CD99 and CagA were negative (Figure 4). The diagnosis of SPTP was finally confirmed and no adjuvant therapy was needed.
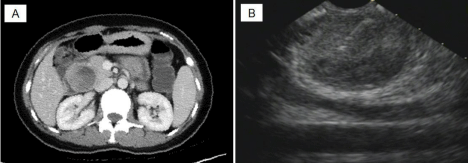
Figure 1: (A) CT revealed a large mass composed of solid and cystic components located on the head of the pancreas. (B) EUS revealed a round mass on the
pancreatic head (FNA-needle can be seen in solid components of tumor).
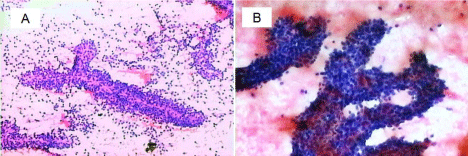
Figure 2: Cell block showed hypercellular nests of ductal epithelial cells with branching papillary features and a central fibrovascular core (A) (H & E x 40) and
adenoid structures composed of cuboidal neoplastic cells (B) (H&E x 100).
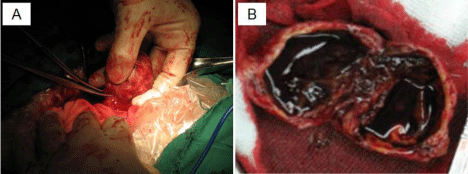
Figure 3: Gross specimen showed around solid mass (A) with cystic change due to hemorrhage and necrosis (B).
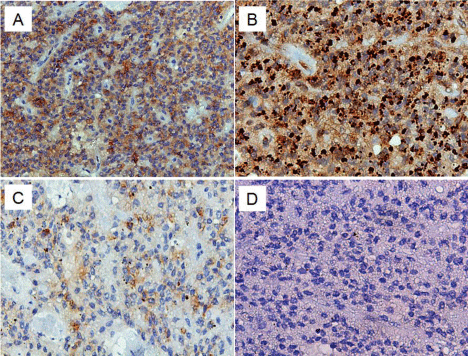
Figure 4: Immunohistochemistry images: Diffusely positive β-catenin (A) (x
40) and vimentin (B) (x 100). Focally positive syn (C) (x 100) and negative
CagA (D) (x 100).
Discussion
SPTP is one of the rare primary tumors of the pancreas. SPTP accounts for 1% of all pancreatic tumors and 3% of all cystic pancreatic neoplasms [4]. SPTP predominantly affects young females (mean age of 25 to 35 years) [1,5] and rarely undergoes metastasis (20%) [6]. Male to female SPTP incidence ration ranges from 1:8 to 1:9 [7,8]. The tumor is thought to be influenced by hormones and their receptors [9-12]. Most patients with SPTP are asymptomatic, as reported in this case. The growth of SPTP is gradual. The mean size of the tumor upon diagnosis is 9.5 cm [13,14]. The tumor is often located in the body and tail of the pancreas (64%) [14]. Clinically, when the mass is greater than 5 cm, patients may present with unspecific symptoms [6], such as bellyache, abdominal mass with discomfort, anorexia, and weight loss. In this case report, SPTP was found in a young female patient, which was considered typical. However, the tumor was found in a less frequent location of SPTP [1]. The value of tumor markers are limited in the diagnosis of SPTP In a Chinese study, alpha fetoprotein, CEA, CA19-9, CA125, and CA242 were found to be slightly increased in 11 out 553 cases of SPTP [8]. Schlitter AM et al. reported that recurrent duodenal ulcer bleeding led to severe anemia in a 17-year-old girl with SPTP [15]. In our case, the patient had mild anemia and CA125 was slightly higher than the normal limit. Aside for SPTP, no other evidence was found by CT or endoscopy to explain the abnormality.
Radiologic findings are important in the diagnosis of SPTP. Procacci et al. [16] reported that CT allowed correct characterization of only 60% of cystic pancreatic masses. Typical SPTP is usually a large encapsulated mass with heterogeneous intensity, frequently showing wide hemorrhage and cystic changes [17,18]. MRI can be utilized in the differential diagnosis of complex cystic masses within the pancreas [19]. Nowadays, EUS is considered as a more accurate diagnostic modality for pancreatic tumor, especially for tumors less than three centimeters [20]. Most cases of SPTP require cytologic diagnosis [21]. An accurate pre-operative diagnosis obtained by EUS-FNA, which is a minimally invasive and helpful method used to diagnose SPTP. Accurate pre-operative diagnosis enables minimal surgery to preserve the pancreas. Previous studies reported that the cytology features of specimens obtained by EUS-FNA and immunohistochemistry provides a more accurate diagnosis of SPTP [22,23]. In this case, we used a 22-gauge needle to obtain the aspirated specimens. The cytologic features of the aspirate specimen were characteristic compared with other cystic or solid tumors of the pancreas. The most typical description of SPTP is the presence of cellularity with branching papillary features composed of fibrovascular core lined with one or more layers of tumor cells [21,24,25]; similar to what was found in the specimen from our case. Immunohistochemically, most SPTPs are strongly positive for vimentin [26,27], NSE, and CD10 [27] and focally positive with syn [28]. However, NSE or CD10 has a low specificity for SPTPs because both are positive in other neoplasms [27]. PR is positive in many SPTPs. SPTP diagnosis can be confirmed when CD10 and PR are both positive [29]. CD56 expression was positive in 55% to 100% of SPTP cases [30].
Conclusion
EUS-guided FNA is a new approach to diagnose SPTPs accurately. EUS is a minimally invasive and safe method to diagnose SPTP with typical cytomorphologic and immunohistochemical features prior to surgical excision.
References
- Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, et al. Cystic neoplasms of the pancreas and tumor like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004; 445: 168-178.
- Frago R, Fabregat J, Jorba R, Garcia-Borobia F, Altet J, Serrano MT, et al. Solid pseudopapillary tumors of the pancreas: diagnosis and curative treatment. Rev Esp Enferm Dig. 2006; 98: 809-816.
- Frantz VK. Tumor of the pancreas. In: Atlas of Tumor Pathology: Fascicles 22 and 28, Section 7. Washington DC: US Armed Forces Institute of Pathology. 1959; 32-33.
- Yang F, Fu DL, Jin C, Long J, Yu XJ, Xu J, et al. Clinical experiences of solid pseudopapillary tumors of the pancreas in China. J Gastroenterol Hepatol. 2008; 23: 1847-1851.
- Brazdil J, Hermanova M, Kren L. Solid pseudopapillary tumor of the pancreas: 5 case reports. Rozhl Chir. 2004; 83: 73-88.
- Casadei R, Santini D, Calculli L, Pezzilli R, Zanini N, Minni F. Pancreatic solid-cystic papillary tumor: clinical features, imaging findings and operative management. JOP. 2006; 7: 137-144.
- Klimstra DS, Adsay NV. Tumors of the pancreas and ampulla of Vater. Odze RD, Goldblum JR, editors. In: Surgical pathology of the GI tract, liver, biliary tract and pancreas. 2nd Edn. Philadelphia: WB Saunders. 2009; 944-946.
- Yu PF, Hu ZH, Wang XB, Jian-Min Guo, Xiang-Dong Cheng, Yun-Li Zhang, et al. Solid pseudopapillary tumor of the pancreas: A review of 553 cases in Chinese literature. World J Gastroenterol. 2010; 16: 1209-1214.
- Ladanyi M, Mulay S, Arseneau J, Bettez P. Estrogen and progesterone receptor determination in the papillary cystic neoplasm of the pancreas. With immunohistochemical and ultrastructural observations. Cancer. 1987; 60: 1604-1611.
- Kosmahl M, Seada LS, Janig U, Harms D, Kloppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000; 436: 473-480.
- Costa SRP, Henriques AC, Godinho CA. Pancreatic solid-cystic papillary tumor: clinical aspects, radiological findings and surgical treatment in a series of five patients. Einstein. 2007; 5: 161-165.
- Mohammadi TA, Rahmani SH, Mozaffar M. Solid pseudopapillary tumor of pancreas: presentation and management. Shiraz E-Med J. 2008; 9: 149-157.
- Chen X, Zhou GW, Zhou HJ. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas. Hepatobiliary Pancreat Dis Int. 2005; 4: 456-459.
- Thompson LDR, Heffess CS. Pancreas. Mills SE, Carter D, Greenson JK, Reuter VE, Stoler MH, editors. In: Diagnostic surgical pathology. 5th Edn. Philadelphia: Lippincott Williams & Wilkins. 2010; 1469-1471.
- Schlitter AM, Konukiewitz B, Kleeff J, Kloppel G, Esposito I. Recurrent duodenal ulcer bleeding as the first manifestation of a solid pseudopapillary neoplasm of the pancreas with hepatic metastases. Dtsch Med Wochenschr. 2013; 138: 1050-1053.
- Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999; 23: 906-912.
- Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996; 199: 707-711.
- Hu S, Lin X, Song Q, Chen K. Multidetector CT of multicentric solid pseudopapillary tumor of the pancreas: a case report and review of the literature. Cancer Imaging. 2011; 11: 175-178.
- Cantis Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol. 2003; 181: 395-401.
- Muller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994; 190: 745-751.
- Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma P, Insabato L. Solid-pseudopapillary tumor of the pancreas: a neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol. 2002; 27: 325-334.
- Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, et al. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Acta Cytol. Endoscopy. 2008; 40: 200-203.
- Chatzipantelis P, Salla C, Apostolou G, Christodoulou L, Kakiopoulos G, Patralexis C, et al. Endoscopic ultrasound-guided fine needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas: a report of 3 cases. Acta Cytol. 2010; 54: 701-706.
- Pettinato G, Manivel JC, Ravetto C, Terracciano LM, Gould EW, di Tuoro A, et al. Papillary cystic tumor of the pancreas. A clinicopathologic study of 20 cases with cytologic, immunohistochemical, ultrastructural, and flow cytometric observations, and a review of the literature. Am J Clin Pathol. 1992; 98: 478-488.
- Katz LB, Ehya H. Aspiration cytology of papillary cystic neoplasm of the pancreas. Am J Clin Pathol. 1990; 94: 328-333.
- Ozkara S, Vardar Aker F, Yitik A, Tosun I, Sagla A, Akkan Cetinkaya Z, et al. Solid pseudopapillary tumor of the pancreas: a case series of 9 patients. Turk J Gastroenterol. 2012; 23: 727-735.
- Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008; 39: 251-258.
- Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, Guiter G, et al. Endoscopic ultrasound-guided fineneedle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol. 2004; 121: 654-662.
- Serra S, Chetty R. An immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol. 2008; 61: 1153-1159.
- Tiemann K, Heitling U, Kosmahl M, Kloppel G. Solid pseudopapillary neoplasms of the pancreas show an interruption of the Wnt-signaling pathway and express gene products of 11q. Mod Pathol. 2007; 20: 955-960.
