Abstract
Adult Diffuse Large B Cell Lymphoma (DLBCL) is a heterogeneous form of hematopoietic cancer and difficult to treat. In order to find a better diagnostic indication for the disease, we analyzed Low Molecular Weight Protein Tyrosine Phosphatase (LMWPTP) that in humans is encoded by the ACP1 gene. LMWPTP is an enzyme shown to counteract Protein Tyrosine Kinases (PTK) and was suggested to be a negative growth factor regulator. However, the 18 kDa PTP can also have a positive effect on cell growth and proliferation, indicating a controversial role in the tumorigenic process. LMWPTP exists in different isoforms which are electrophoretically, kinetically and immunologically distinct. We have studied two subgroups of DLBCL consisting of a Germinal Center B cell like (GCB) and a non-Germinal Center B cell like (non-GCB) group. The two subgroups have been defined by gene-expressing profiling and are associated with differential outcome. The expression levels of LMWPTP protein was compared and showed significant differences between the GCB and non- GCB subgroups (p=0.012). Interestingly, when the samples were divided into survivors and non-survivors, and thereafter analyzed for LMWPTP expression, the samples from patients with a higher survival rate showed increased staining intensity, whereas the samples from patients with lower intensity of LMWPTP did not survive the disease (p=0.001). In conclusion, we have shown that DLBCL patients with worse outcome express LMWPTP with a lower intensity, suggesting a tumor suppressor role for this form of the enzyme.
Keywords: ACP1; B cell; DLBCL; Germinal center; LMWPTP; Lymphoma; Non-germinal center; Prognosis
Introduction
The levels of protein phosphotyrosine in signaling molecules are strictly regulated by Protein Tyrosine Phosphatases (PTP) and Protein Tyrosine Kinases (PTK), controlling cell growth responses including proliferation, differentiation, adhesion, migration, metabolism and cytoskeletal function [1,2]. The low molecular weight 18 kDa PTP enzyme LMWPTP is encoded by the acid phosphatase locus 1 gene ACP1, and has been implicated in cell growth regulation, cytoskeleton rearrangement and immune regulation. The human ACP1 gene is genetically polymorphic and has three alleles, namely A, B and C, giving rise to six genotypes [3,4]. Out of five translated proteins, two correspond to the main active isoforms, which are the distinct isoforms based on their electrophoretic mobility, termed fast and slow [5,6]. These isoforms arise from mutually exclusive alternative splicing of either exon 3 or 4, and their protein sequence differs by a 42-amino acid internal sequence [7]. LMWPTP interacts with growth factor receptors and proliferation signaling pathways, and it is regulated by phosphorylation/dephosphorylation or oxidation of the protein [2,3,8]. Two conserved tyrosines Tyr 131 and Tyr 132 in LMWPTP regulate adhesion of the enzyme by a balance of alternative phosphorylation performed mainly by Src kinases [9,10]. LMWPTP has been shown to interact with molecules such as Platelet- Derived Growth Factor Receptor (PDGFR), Janus kinase 2 (Jak2), the signal transducer and activator of transcription 5 (STAT5), β-catenin, ephrin type-A receptor 2 (EphA2) and focal adhesion kinase (Fak) [11-15], resulting in a positive effect on cell growth and proliferation [10,16]. LMWPTP has also been described as a negative regulator of cellular proliferation induced by growth factors. Souza et al suggested that active (reduced) or inactive (oxidized) forms of LMWPTP play important roles in cancer cell signaling: the reduced form can be involved in transformation (EphA2, loss of adhesion), whereas the oxidized form can promote survival pathways through activation of JAK2 and STAT5 [8]. Moreover, LMWPTP was implicated in predicting malignant potential in prostate cancer outcome and in mediating malignant potential in colorectal cancer [17,18].
Diffuse Large B Cell Lymphoma (DLBCL) represents the most common subtype of Non-Hodgkin Lymphoma (NHL) worldwide, accounting for up to 40% of all newly diagnosed cases [19]. Importantly, DLBCL is readily curable with immunochemotherapy in the majority of patients, even in the most advanced cases. Moreover, recent advances in molecular genetics may be selectively exploited, allowing for a more effective and personalized approach to treatment [19]. Gene expression profiling have identified distinct molecular subtypes, termed Germinal Center B cell (GCB) like and activated B cell (ABC), or non-germinal center B cell (non-GCB) like, representing lymphomas arising from different stages of lymphoid differentiation [20, 21]. The molecular subtypes represent lymphomas that are driven by very different intracellular oncogenic signaling pathways that could be differentially exploited for therapeutic benefit.
We have investigated paraffin-embedded samples from 28 patients with de novo DLBCL using immunohistochemistry. In this study, two prognostic subgroups of DLBCL were divided by the use of immunohistochemistry in a GCB and a non-GCB group, based on the expression of CD10, Bcl6 and IRF according to previous studies [22-24]. The intensity of LMWPTP expression could be significantly distinguished between the two subgroups. Moreover, when the samples were divided in survivors and non-survivors, patients with increased LMWPTP staining intensity showed a higher survival rate, whereas the samples from patients with lower staining intensity did not survive the disease.
Materials and Methods
Tissue array and immunohistochemistry
As previously described, a total of 28 pieces of paraffin embedded de novo DLBCL tissues from 8 men and 20 women with a median age of 70 and diagnosed between 2001 and 2006, were selected by a pathologist from the Department of Pathology, University Hospital in Skane, Malmö, Sweden (Ethical approvement No. LU 210/ 2006) [22- 24]. Of 28 stained tissue sections, 25 could be evaluated. For controls, human tonsillar tissue was used. Representative areas in all paraffin blocks were chosen and tissue arrays were constructed as described earlier [22]. In brief, punches of 0.6 mm were taken from each block and mounted manually in a recipient block. Four micrometer sections were dried, deparaffinized, rehydrated and treated in a microwave for 10 min with target retrieval solution pH 9.9. This was followed by incubation in an automated immunohistochemical staining machine (Techmate 500, Dako, Copenhagen, Denmark) with primary antibodies: mouse anti-human CD10 (Serotec, Oxford, UK), mouse anti-human Bcl6 (Chemicon/Millipore, Bedford, MA), mouse antihuman MUM1/IRF4 (Abcam, Cambridge, UK) and rabbit antihuman ACP-1; HPA016754 (Sigma-Aldrich, St Louis, MO). Dako real envision detection system peroxidase/DAB (Dako, Copenhagen, Denmark) was used for incubation with secondary antibodies and visualization. Slides were counterstained with haematoxylin. Analyses were performed independently by one pathologist and two researchers. There was a 90% agreement between the observers, and in cases of variance, the pathologist determined the outcome. To classify patients into the GCB- or non-GCB group, we used the three marker-model previously described in Fridberg et al. [22]. In brief, cases were considered GCB-positive if CD10 alone or both CD10 and Bcl6 were positive. If both CD10 and Bcl6 were negative, the cases were considered GC-negative. If CD10 was negative and Bcl6 positive, IRF4 determined the outcome: Positivity for IRF4 designated the patient material to the non GCB-group while negative IRF4 staining meant the GCB group. The staining intensity for LMWPTP was graded from 0 to 3. 0=no staining, 1=weak staining, 2=moderate staining and 3=strong staining. For measuring the fraction of positive cells, we used a 25% cut off to classify patients as positive or negative for all markers. Further, proportion of tumor cells less than 25% positive were scored as 1; greater than 25%, but less than 50% positive were scored as 2; greater than 50%, but less than 100% positive were scored as 3. Statistical calculations were performed using SPSS.
Results
The 28 patient samples were divided into a GCB- and a non- GCB group based on the expression of GCB markers CD10, Bcl6 and post-GC marker IRF4 as analyzed by immunohistochemistry and described in Fridberg et al. [22]. Fourteen patients (4 men, 10 women) were classified as belonging to the GCB group and 14 patients (4 men, 10 women) constituted the non-GCB group. We found that the non-GC group was characterized by a more advanced stage disease (stage III-IV) compared with the GCB group (p-value of 0.022). The staining intensity and percentage positive cells for the anti-human ACP-1 was analyzed in 25 DLBCL samples and three normal tonsillar samples. More of the samples in the GCB group have a higher staining intensity compared to the samples of the non- GCB group. When the both groups were compared, a significant difference could be demonstrated (p=0,012) (Table 1). Samples from three individuals containing normal tonsillar samples were evaluated for LMWPTP staining intensity, and were shown to have moderate staining; intensity 2 and the percentage of stained cells were high, 51-100%. The percentage of stained DLBCL cells for LMWPTP did not differ between the groups (p=0.419) (Table 1). The 25 DLBCL cases analyzed for LMWPTP expression were related to survival and a higher intensity of the staining was found to correlate with increased survival (p=0.001) (Table 2). No such difference could be seen for the percentage of stained cells (p=0.663) (Table 2). The statistical analyses were performed by Mann Whitney tests. The staining intensity of different DLBCL samples is shown in (Figure 1). The figures represent no staining (A), weak staining (B), moderate staining (C) and strong staining (D). One of the three samples of normal tonsillar samples with moderate staining is shown (Figure 2).
ACP1
GCB (n)
%
Non-GCB (n)
%
Intensity
0
0
0
0
0
1
3
21.5
6
54.5
2
8
57
4
36.5
3
3
21.5
1
9
Total
14
100
11
100
P-value
0.012
Percent
0-25 %
0
0
0
0
26-50 %
3
21
4
36
51-100 %
11
79
7
64
Total
14
100
11
100
P-value
0.419
Table 1: Intensity of ACP1 expression and the percentage of stained cells in GCB and non-GCB subgroups of DLBCL.
ACP1
Mors (n)
%
Survivors (n)
%
Intensity
0
0
0
0
0
1
5
56
2
12
2
3
33
8
50
3
1
11
6
38
Total
9
100
16
100
P-value
0.001
Percentage
0-25 %
0
0
0
0
26-50 %
4
44
3
19
51-100 %
5
56
13
81
Total
9
100
16
100
P-value
0.663
Table 2: Correlation of the protein intensity of ACP1 and outcome of DLBCL.
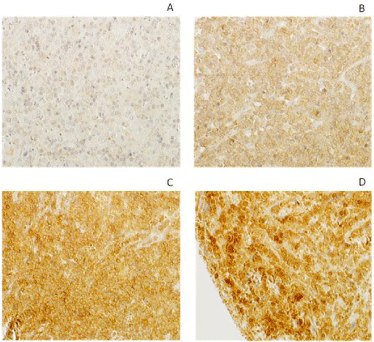
Figure 1: Immunohistochemical staining of de novo DLBCL tissue. A. ACP1 with no staining B. ACP1 with weak staining C. ACP1 with moderate staining D. ACP1
with strong staining.
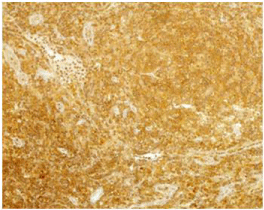
Figure 2: One sample of normal tonsillar samples with moderate staining
with ACP1 is shown.
Discussion
The molecular distinction between the GCB and ABC (non- GCB) like cells has prognostic implications; with the ABC subtype exhibiting an inferior outcome following the most commonly used initial therapy [19]. GCB DLBCLs are believed to derive from lymphoid cells residing in the GC and therefore express genes such as CD10, LMO2, and the transcriptional repressor BCL6 [20,21]. ABC DLBCLs derived from B cells at a plasmablastic stage, just prior to GC exit, express genes that are frequently expressed in mature plasma cells and have increased cell survival, proliferation, and inhibited apoptosis [25,26]. Our results indicate that the non-GCB subgroup with worse outcome, also called ABC, express LMWPTP with lower intensity. This might indicate a tumor suppressor function for LMWPTP.
The role of the enzyme in tumor development and progression is disputed. The type of cancer, the tumor stage, the methodology (PCR, Western blot, immunohistochemistry) and the choice of primers or antibodies for the investigation might be related to the disparity of the outcome of different studies, as well as the existence of the two isoforms of ACP1, the fast and the slow isoform. Upregulation and regulation of the fast isoform of ACP1 has been demonstrated in breast, cervix and colon cancer [4,27,28]. Interestingly, the fast isoform is related with cytoskeletal and cellular organization, as well as spreading. When investigating breast cancer cell lines, Alho et al. proposed that the two LMWPTP isoforms have opposing roles in the tumorigenic process in breast, with the slow isoform being oncogenic and the fast isoform antioncogenic, which can explain the previous contradictory findings regarding the role of LMW-PTP in cancer [29]. Malentacchi et al. showed that LMWPTP mRNA is increased in colon cancer and neuroblastoma without any difference in the isoforms expressed [30], and in animal models, LMWPTP acts as a positive regulator of tumor onset and growth [15]. Recently, LMWPTP was also implicated in predicting malignant potential in prostate cancer outcome [16] and in mediating malignant potential in colorectal cancer [17]. The postulated oncogenic role of ACP1 is contradictory to our results, and to others that have shown that suppression of LMWPTP enhances migration of mammary epithelial cells [31]. Expression of the slow isoform can be protective, at least in colon cancer [27], and is located in the cytoplasm, whereas the fast isoform has a cytoskeletal location [32]. Interestingly, the sequence of the fast and slow isoforms that differs by a 42-amino acid internal sequence [7] is located in a loop that flanks the catalytic active site and can determine isoform specificity in the binding to substrates and modulating ligands [33].
Conclusion
We have shown that the intensity of the LMWPTP protein expression differs significantly between the GCB and non-GCB subgroups. Moreover, patients with a higher survival rate show increased staining intensity, whereas the samples from patients with lower intensity do not survive the disease. The next step would be to map the epitopes for the different LMWPTP antibodies used in investigations, since the isoforms differ by a 42-amino acid internal sequence. Also, mRNA for slow and fast isoforms in DLBCL should be analyzed to find out whether there is a balance favoring the expression of either isoforms.
Acknowledgement
This work was supported by grants from Malmo University.
References
- Alonso A, Pulido R. The extended human PTPome: a growing tyrosine phosphatase family. FEBS J. 2016; 283: 1404-1429.
- Ruela-de-Sousa RR, Queiroz KC, Peppelenbosch MP, Fuhler GM. Reversible phosphorylation in haematological malignancies: potential role for protein tyrosine phosphatases in treatment? Biochim Biophys Acta. 2010; 1806: 287-303.
- Raugei G, Ramponi G, Chiarugi P. Low molecular weight protein tyrosine phosphatases: small, but smart. Cell Mol Life Sci. 2002; 59: 941-949.
- Alho I, Clara Bicho M, Carvalho R, Da Silva AP, Costa L, Bicho M. Low molecular weight protein tyrosine phosphatase genetic polymorphism and susceptibility to cancer development. Cancer Genetics and Cytohenetics. 2008; 181: 20-24.
- Bryson GL, Massa H, Trask BJ, Van Etten RL. Gene structure, sequence, and chromosomal localization of the human red cell-type low-molecular-weight acid phosphotyrosyl phosphatase gene, ACP1. Genomics. 1995; 30: 133-140.
- Rudbeck L, Dissing J, Lazaruk KD, Sensabaugh G. Human 18 kDa phosphotyrosine protein phosphatase (ACP1) polymorphism: studies of rare variants provide evidence that substitutions within or near alternatively spliced exons affect splicing result. Ann Hum Genet. 2000; 64: 107-116.
- Modesti A, Marzocchini R, Raugei G, Chiti F, Sereni A, Magherini F, et al. Cloning, expression and characterisation of a new human low Mr phosphotyrosine protein phosphatase originating by alternative splicing. FEBS Lett. 1998; 431: 111-115.
- Souza AC, Azoubel S, Queiroz KC, Peppelenbosch MP, Ferreira CV. From immune response to cancer: a spot on the low molecular weight protein tyrosine phosphatase. Cell Mol Life Sci. 2009; 66: 1140-1153.
- Bucciantini M, Chiarugi P, Cirri P, Taddei L, Stefani M, Raugei G, et al. The low Mr phosphotyrosine protein phosphatase behaves differently when phosphorylated at Tyr131 or Tyr132 by Src kinase. FEBS Lett. 1999; 456: 73-78.
- Alho I, Costa L, Bicho M, Coelho C. The role of low-molecular-weight protein tyrosine phosphatase (LMW-PTP ACP1) in oncogenesis. Tumour Biol. 2013; 34: 1979-1989.
- Chiarugi P, Cirri P, Raugei G, Camici G, Dolfi F, Berti A, et al. PDGF receptor as a specific in vivo target for low M(r) phosphotyrosine protein phosphatase. FEBS Lett. 1995; 372: 49-53.
- Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007; 133: 1637-1648.
- Rigacci S, Talini D, Berti A. LMW-PTP associates and dephosphorylates STAT5 interacting with its C-terminal domain. Biochem Biophys Res Commun. 2003; 312: 360-366.
- Taddei ML, Chiarugi P, Cirri P, Buricchi F, Fiaschi T, Giannoni E, et al. Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res. 2002; 62: 6489-6499.
- Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002; 277: 39274-39279.
- Chiarugi P, Taddei ML, Schiavone N, Papucci L, Giannoni E, Fiaschi T, et al. LMW-PTP is a positive regulator of tumor onset and growth. Oncogene. 2004; 23: 3905-3914.
- Ruela-de-Sousa RR, Hoekstra E, Hoogland AM, Souza Queiroz KC, Peppelenbosch MP, Stubbs AP, et al. Low-Molecular-Weight Protein Tyrosine Phosphatase Predicts Prostate Cancer Outcome by Increasing the Metastatic Potential. Eur Urol. 2016; 69: 710-719.
- Hoekstra E, Kodach LL, Das AM, Ruela-de-Sousa RR, Ferreira CV, Hardwick JC, et al. Low molecular weight protein tyrosine phosphatase (LMWPTP) upregulation mediates malignant potential in colorectal cancer. Oncotarget. 2015; 6: 8300-8312.
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015; 125: 22-32.
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403: 503-511.
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. Lymphoma/Leukemia Molecular Profiling Project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346: 1937-1947.
- Fridberg M, Servin A, Anagnostaki L, Linderoth J, Berglund M, Soderberg O, et al. Protein expression and cellular localization in two prognostic subgroups of diffuse large B-cell lymphoma: higher expression of ZAP70 and PKC-beta II in the non-germinal center group and poor survival in patients deficient in nuclear PTEN. Leuk Lymphoma. 2007; 48: 2221-2232.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282.
- Berglund M, Thunberg U, Amini RM, Book M, Roos G, Erlanson M, et al. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005; 18: 1113-1120.
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010; 463: 88-92.
- Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010; 362: 1417-1429.
- Spina C, Sacucci P, Bottini E, Gloria-Bottini F. ACP1 genetic polymorphism and colon cancer. Cancer Genet and Cytogenet. 2008; 186: 61-62.
- Alho I, Costa L, Bicho M, Coelho C. Low molecular weight protein tyrosine phosphatase isoforms regulate breast cancer cells migration through a RhoA dependent mechanism. PLoS One. 2013; 8: 76307.
- Alho I, Costa L, Bicho M, Coelho C. Characterization of low molecular weight protein tyrosine phosphatase isoforms in human breast cancer epithelial cell lines. Anticancer Res. 2013; 33: 1983-1987.
- Malentacchi F, Marzocchini R, Gelmini S, Orlando C, Serio M, Ramponi G, et al. Up-regulated expression of low molecular weight protein tyrosine phosphatases indifferent human cancer. Biochem Biophys res Commun. 2005; 334: 875-883.
- Lin G, Aranda V, Muthuswamy SK, Tonks NK. Identification of PTPN23 as a novel regulator of cell invasion in mammary epithelial cells from a loss-of-function screen of the 'PTP-ome'. Genes Dev. 2011; 25: 1412-1425.
- Cirri P, Chiarugi P, Taddei L, Raugei G, Camici G, Manao G, et al. Low molecular weight protein-tyrosine phosphatase tyrosine phosphorylation by c-Src during platelet-derived growth factor-induced mitogenesis correlates with its subcellular targeting. J Biol Chem. 1998; 273: 32522-32527.
- Maccari R, Ottana R. Low molecular weight phosphotyrosine protein phosphatases as emerging targets for the design of novel therapeutic agents. J Med Chem. 2012; 55: 2-22.
Citation: Stanezai S, Sahlen E, El-Schich Z, Fridberg M, Fredrikson GN, Anagnostaki L, et al. Higher Intensity of Low Molecular Weight Protein Tyrosine Phosphatase/ ACP-1 in Survivors of Patients Diagnosed with Diffuse Large B Cell Lymphoma (DLBCL) Compared to Non-Survivors. Austin Biol. 2016; 1(2): 1009.
