Abstract
In the last years, alternatives to animal testing were applied, aimed at reduce the laboratory use of animals, in agreement with the 3Rs principles (Reduction, Refinement and Replacement). In the approach to the study of neurological disorders, several in vitro models and alternative animal species like mammals with a more complex nervous system have been proposed as translational model for human. Lately, canines have been considered an excellent model for study on neurodegenerative disorders since they may develop central nervous system disease close to those observed in humans. In the present report, we describes a method to obtaining primary cultures from dog fetuses brain collected accidentally and cryopreserved until the use. The brain tissues were dissociated through a mild enzymatic papain system and cultured, and the development, morphology and the amount of neuronal cells were assessed. We appreciated the capability of cryopreserved brain tissues to provide a vital and proliferating neuronal primary culture. Moreover, we noticed which explants from early gestation days fetuses (25 days) yielded the higher number of neurons in comparison with those obtained from older fetuses (45days). Our data may suggest a good alternative experimental approach to obtain a primary neuronal culture, without necessarily having to use experimental animals.
Keywords: Fetal dog brain; 3Rs principles; Papain dissociation system; Neurons; β III-Tubulin; Primary cultures
Introduction
Neurodegenerative disorders are heterogeneous and very complicated processes, characterized by altered morphology, impaired functions and loss of neuronal cells [1,2]. In the experimental approach to the study of neurological disorders, two issues seem to be crucial: 1) the experimental model; 2) the species used in the experimental model. As far as the experimental model is concerned, through the years, several in vivo, ex vivo, and in vitro models have been generated, in order to better understand crucial aspects on neurodegeneration [3,4]. Over the past decade, EU directives have aimed at limiting the use of experimental animals (3Rs principles) strongly encouraging an increase in in vitro experiments [5,6], which compared to in vivo models appear to be considerably more advantageous in the field of neurological disorders. In fact, by using in vitro models, it is possible to study the role of isolated cells of one particular type in an environment that simulates the disease and to investigate mechanisms of a possible deleterious or protective role of specific molecules and compounds. Tissue culture of dissociated cells is considered to be a useful method for examining the function and development of the cells from both the central and peripheral nervous system [7]. As regards the species employed in the experimental protocols, unfortunately the study of neurodegenerative diseases lacks of a complete translational model, thus resulting in many animal models being used, ranging from rodents to non-human primates, each with its advantages but also associate challenges [8]. In general, although rodents remain the most widely used species for human neurodegenerative study, other mammals may also be useful as they flaunt a more complex anatomy and physiology, making them more comparable to humans for several aspects [9-11]. In the last years, canines have been considered an excellent model for studies on neurodegenerative disorders. In an excellent review, Starkey [12] pointed out the many advantages of using dogs as a translational model for human medical research. In particular, the canine nervous system is much more similar in size to that of humans than that of rodents. Moreover, researches have demonstrated that dogs suffer from a number of diseases which may overlap with those observed in the human brain such as cortical atrophy, neuronal loss, amyloid deposition in the brain vessels and hereditary neurodegenerative disorders [13-18]. Setting up experimental in vitro protocols starting from the cellular elements closest to the species of which we want to study certain diseases, often poses problems related to the obtainment of the biological material, especially considering the scarcity of both cell types and species on the market. In the last years authors have demonstrated that it is possible to take advantage of organic parts of particular species intended for destruction, picked up from public slaughterhouses for example, from which viable and cultivable cellular elements can be obtained [10,19]. The aim of the present work is therefore to describe an easy way of obtaining and characterizing a primary culture from dog foetal neurons, using an experimental approach in agreement with the 3R principles, considering the growing interest on the study of neurodegenerative pathologies of the dog as a model for human and solving the problem concerning the scarcity of dog neuronal cell lines in the commerce.
Materials and Methods
Sampling
The material (foetal dog brain) used for the primary cell culture was collected thanks to the collaboration between the Normal Anatomy Section and the Clinical Obstetrics of the Veterinary Teaching Hospital of our department. The hospital, in accordance with the Italian law, has an agreement with the municipal dog pound and the Public Health Offices, to ensure the sterilization of stray bitches thus trying to prevent an increase of stray dogs in the city. Whenever pregnant uteri were found during ovariohysterectomy surgical procedures, they would be excised, immediately refrigerated and provided to our laboratory. Once the uterus is opened, under sterile conditions, we proceed to the foetal adnexa dissection and the removal of fetuses. The foetal age was assessed by crown-rump length measurement and specific tables [20]. By dissecting the head under a stereo microscope, it brought out the brain tissue trying to free him as much as possible from the meninges. Following the procedures illustrated by [11], fragments from each foetal brain were suspended in an ice-cold cell freezing medium composed of Dulbecco’s Modified Eagle’s Medium (DMEM) with 1% HEPES (pH 7.4), 1% foetal calf serum and 10% Dimethylsulfoxide (DMSO). Cryovials were cooled to -80°C and then transferred to liquid nitrogen and stored until use.
Cell culture procedures
For the preparation of primary neuronal cultures, we used a mild enzymatic dissociation procedure with a Papain dissociation system (Worthington Biochemical Corp., Lakewood, NJ), following methodologies yet reported in literature [21,22]. Basically, frozen foetal brain tissues were rapidly thawed in a water bath at 37°C, grossly minced in small pieces and dissociated. The brain tissue was treated with Earle’s Balanced Salt Solution (EBSS) containing papain and DNase I (DNase) for 40 minutes at 37°C, centrifuged at 1000 rpm for 5 minutes. Once the cell suspension was layered on top of EBSS containing albumin-inhibitor in a centrifuge tube, it was then centrifuged at 800 rpm for 6 minutes. The supernatant was discarded, and the pelleted cells were resuspended in the culture medium Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/ F12; Euroclone, Milan, Italy) supplemented with penicillin (30 mg/L) with streptomycin (50 mg/L), sodium bicarbonate (2.4 g/L), insulin (10 μg/mL), transferrin (10 μg/mL), sodium selenite (10-8M), 10% foetal calf serum. Moreover, as also shown by other authors [23], in the same medium we added the following specific growth factors: 100 ng NGF (Nerve Growth Factor- Sigma, St. Louis, MO, USA), 10 ng/ ml GDNF (Glial Cell Line Derived Neurotrophic factor- Sigma, St. Louis, MO, USA) and 2 ng/ml of BDNF (Brain Derived Neurotrophic Factor- Sigma, St. Louis, MO, USA), in order to promote and enhance growth of neuronal cells. Cells were plated at the rate of 5×105 into about 10 Petri dishes with cover-glass slide on the bottom previously coated with polylysine (Sigma, St. Louis, MO, USA), and placed in incubator (37°C; 5% CO2). Half of the culture medium was changed every 2-3 days.
Immunofluorescence staining
Cells were fixed in cold 100% methanol for 20 min. After washing with PBS, fixed cells were incubated overnight with the following antibodies diluted with PBS containing 3% bovine serum albumin (BSA) and 0,1% tryton X-100: anti-β III tubulin (monoclonal and polyclonal, Sigma, St. Louis, MO, USA); anti-vimentin (monoclonal, 1:1,000, Sigma, St. Louis, MO, USA). After rinsing with PBS, cells were incubated for 1 hr at 37°C with secondary anti-rabbit and antimouse fluorescein/tetramethylrhodamine isothiocyanate-conjugated antibodies (FITC-AlexaFluor 488, TRITC-AlexaFluor 594, 1:400, Invitrogen, Carlsbad, CA, USA). All images were obtained with a confocal laser scanning microscope from Leica (TCS SP5 DMI 6000CS, Leica Microsystems GmbH, Wetzlar, Germany) using a 40/60X oil objective. FITC was exited at 488 nm and the emission was detected between 510 and 550 nm, whereas Rhodamine was excited at 568 nm and emission was detected between 585 and 640 nm. Nuclear counterstaining was performed using Hoescht blue-33342 (1:5,000, Sigma, St. Louis, MO, USA). The estimation of β III-tubulin positive cells number was performed under the confocal microscope by counting at magnification of x40 in 10 randomly chosen fields.
Statistical Analysis
Differences between groups were analyzed by Student’s t test. Differences were considered significant when P<0.05.
Results
Sampling
The procedures for obtaining dog fetuses, did not allow homogeneous sampling. In fact, the finding of pregnant uteri was an accidental event, detected only at the time of surgery, since no previous instrumental investigation to diagnose pregnancy was carried out. This led to the collection of brain tissues belonging to fetuses at different stage of foetal development. In particular, at the end of our sampling we collected mainly fetuses of 25 (Figure 1) and 45 of gestation days in average. This also affected the characteristics of our cultured cells, in terms of numbers.

Figure 1: Canine embryo at approximately 25 gestation days.
Cell cultures and neuronal identification
Once seeded, cells appeared with a round shape and clustered (Figure- 2 A,B,C). The adhesion to the bottom of the petri plate was well evident between the first and the second day of culture. At first, the medium appeared with a certain turbidity which improved in the following days, with partial changes of medium, performed every two days on average. The first extension, characteristic of neural cells, was evident around the fourth/sixth day of culture (Figure-2D), well detectable around the 15 days from seeding (Figure-2E,F). The β III-tubulin immunostaining revealed that the highest percentage of cells was neuronal cells that were obtained in cultures derived from 25 gestation days fetuses (Figure-3A). On the contrary, the lower percentage of neurons was obtained from a brain of 45 gestation days fetuses (Figure-3B). Moreover, in the cultures of cells obtained from the oldest fetuses, we found a discrete number of cells unstained by β III-tubulin antibody, which then resulted positive to vimentin antibody (Figure-3C).
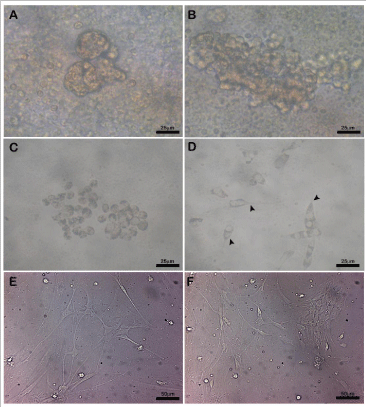
Figure 2: Phase -contrast optics images of dog brain cells in primary culture.
Appearance at: the same day of the seeding (A, B), four days (C), six days
with a well detectable changes in neuronal shape with the elongation of
characteristic cell processes (D, black head arrows ) and fifteen-eighteen
days from the seeding (E, F) with more evident phenotypic neural features.
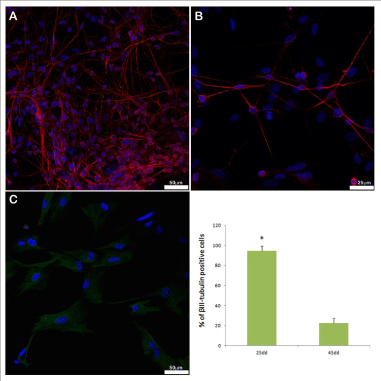
Figure 3: Confocal images showing immunocytochemical β III-tubulin
detection in primary neural cultures from 25 (A) and 45 (B) gestation days
fetuses, respectively. Vimentin (C) positive cells founded in culture from 45
gestation days fetuses. The diagram shows the quantification of the amount
of β III-tubulin positive cells in cultures from brain explants of both 25 and
45 gestation days fetuses. * indicates P<0.005. Each bar represents mean
± SEM.
Discussion
In the last decades, alternatives to animal testing have been proposed in order to enforce the strategy of 3Rs principles for reduction, refinement and replacement of laboratory use of animal [5,24]. In searching large animal models that share more similarities with the human kind, the dog proved to be an excellent translational model [8,25], mimicking important aspects of human brain anatomy, physiology and pathology. It is true that canine cells have already been used, but they had often been obtained either from adult subjects (Primary Olfactory Ensheating Cells) or derived from canine embryostem cells, therefore always obtained from housed experimental animals [26-29]. In this work a procedure for the isolation and primary cultures of canine brain cells is described, in which an original approach is used employing dog fetuses accidentally collected from stray bitches which underwent surgical sterilization procedures. This protocol yielded interesting results, showing several positive aspects but also some disadvantages. First off, one of the most important data was to appreciate the biological material collected which provided a significant amount of cultivable neural cells, confirming what had already been shown by other authors in relation to the usefulness of biological material usually destined for destruction [10,30,31]. As far as sampling and storage procedures are concerned, the results have confirmed the usefulness of cryopreservation of explanted material. This is a peculiar aspect of this type of experimental protocol since it is characterized by a sampling of accidental type, where it is essential to be able to store the material as soon as it can be collected so that it can be safely used at a later time. Several authors have already described this procedure for the brain tissues from other species such as bovine, rats, primates and humans [32-35]. In this study, it has been observed that the cryopreservation of the neuronal tissues explanted has in no way affected the capacity for growth and differentiation of neural cells obtained, corresponding to what has previously described. Concerning the methods employed for the setting of cell cultures the use of a mixed protocol, with mechanical trituration coupled to a not too aggressive papain digestion has allowed to obtain a high number of proliferating cells, confirming the usefulness of the papain for dissociation of the cells of the nervous system [7,36]. Moreover, the addition in the medium of growth factors (BDNF, NGF, GDNF) specific for neuronal cells has proven to be highly effective, significantly favouring the growth and differentiation of neuronal cells as shown in previous works [22,23,37]. Unfortunately a protocol like ours, based on an “accidental” sampling, presented some limitations. Unlike small experimental animals, canine fetuses are much less available and more difficult to procure, thus not allowing a homogeneous sampling depending on the gestation age. Our sampling in fact, led to the collection of brain material from fetuses of two main gestation periods i.e. 25 and 45 gestation days. The different ages of the fetuses have affected the quality and the growth capacity of our cell culture. In fact, the number of β III-tubulin positive cells obtained from the fetuses of early gestation days (25days) in comparison with those obtained from the brain tissues belonging to 45 gestation days fetuses, where more abundant. Moreover, in culture from 45 gestation days fetuses, other cells vimentin positive (fibroblasts) were found. This was in line with what was demonstrated in previously published works which reported that the foetal age may influence the amount of neurons, and the cell proliferation capability, which are greater with early gestation age fetuses [11,38].
Conclusions
Summing up, although we are neither conscientious objectors nor against using experimental animals, with the present investigation we have shown the possibility of obtaining a primary culture, viable and proliferating, starting from a foetal brain, which was collected accidentally. This could represent a useful alternative to experimental methods, in line with European directives, which are more and more oriented towards the replacement of experimental animals. Moreover, the use of biological material from animals with a more complex brain may be useful to set up experimental protocols for studying pathophysiological aspects useful both for that particular species and the human species. In our specific case, we could take advantage of a species, like the dog, which is increasingly attracting attention as a translational model for human neurodegenerative disorders.
Acknowledgement
The author is grateful to Mrs. Nicole Nasgowitz for the revision of the English language of the manuscript.
This work was supported by grant CRP-25879/2011, from the Sardinia Region Research Support.
References
- De Pedro-Cuesta J, Rábano A, Martínez-Martín P, Ruiz-Tovar M, Alcalde-Cabero E, Almazán-Isla J, et al. Comparative incidence of conformational, neurodegenerative disorders. PLoS One. 2015; 10: 137342.
- Hardy J, Revesz T. The spread of neurodegenerative disease. N Engl J Med. 2012; 366: 2126-2140.
- Yildiz-Unal A, Korulu S, Karabay A. Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatr Dis Treat. 2015; 5: 297-310.
- Schlachetzki JC, Saliba SW, Oliveira AC. Studying neurodegenerative diseases in culture models. Rev Bras Psiquiatr. 2013; 35: 92-100.
- Doke SK, Dhawale SC. Alternatives to animal testing: A review. Saudi Pharm J. 2015; 23: 223-229.
- Balls M. Alternatives to animal experimentation yesterday, today and tomorrow: some thoughts on leaving ECVAM. Altex. 2002; 19: 225-227.
- Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986; 6: 3044-3060.
- Head E. Neurobiology of the aging dog. Age. 2011; 33: 485-496.
- Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012.
- Hashimoto A, Onodera T, Ikeda H, Kitani H. Isolation and characterization of fetal bovine brain cells in primary culture. Res Vet Sci. 2000; 69: 39-46.
- Richard O, Duittoz AH, Hevor TK. Early, middle, and late stages of neural cells from ovine embryo in primary cultures. Neurosci Res. 1998; 31: 61-68.
- Starkey MP, Scase TJ, Mellersh CS, Murphy S. Dogs really are man's best friend--canine genomics has applications in veterinary and human medicine!. Brief Funct Genomic Proteomic. 2005; 4: 112-128.
- Thomas R, Duke SE, Wang HJ, Breen TE, Higgins RJ, Linder KE, et al. ‘Putting our heads together’: insights into genomic conservation between human and canine intracranial tumors. J. Neurooncol. 2009; 94: 333-349.
- Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2009; 106: 2794-2799.
- Dimakopoulos AC, Mayer RJ. Aspects of neurodegeneration in the canine brain. J Nutr. 2002; 132: 1579-1582.
- Nakamura S, Tamaoka A, Sawamura N, Kiatipattanasakul W, Nakayama H, Shoji S, et al. Deposition of amyloid beta protein (A beta) subtypes [A beta 40 and A beta 42(43)] in canine senile plaques and cerebral amyloid angiopathy. Acta Neuropathol. 1997; 94: 323-328.
- Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996; 17: 259-268.
- Cummings BJ, Su JH, Cotman CW, White R, Russell MJ. Beta-amyloid accumulation in aged canine brain: a model of early plaque formation in Alzheimer's disease. Neurobiol Aging. 1993; 14: 547-560.
- Peruffo A, Cozzi B. Bovine brain: an in vitro translational model in developmental neuroscience and neurodegenerative research. Front Pediatr. 2014; 2: 1-4.
- McGeady TA, Quinn PJ, Fitzpatrick ES, Ryan MT, Cahalan S. Veterinary Embryology. Oxford: Wiley-Blackwell. 2006.
- Akagi T, Mandai M, Ooto S, Hirami Y, Osakada F, Kageyama R, et al. Otx2 homeobox gene induces photoreceptor-specific phenotypes in cells derived from adult iris and ciliary tissue. Invest Ophthalmol Vis Sci. 2004; 45: 4570-4575.
- Gill JC, Moenter SM, Tsai PS. Developmental regulation of gonadotropin-releasing hormone neurons by fibroblast growth factor signaling. Endocrinology. 2004; 145: 3830-3839.
- Duittoz AH, Hevor T. Primary culture of neural precursors from the ovine central nervous system (CNS). J. Neurosci Methods. 2001; 107: 131-140.
- Ranganatha N, Kuppast IJ. A review on alternative to animal testing methods in drug development. Int J Pharm Pharm Sci. 2012; 4: 28-32.
- Head E, Thornton PL, Tong L, Cotman CW. Initiation and propagation of molecular cascades in human brain aging: insight from the canine model to promote successful aging. Prog Neuropsychopharmacol Biol Psychiatry. 2000; 24: 777-786.
- Gerhauser I, Hahn K, Baumgärtner W, Wewetzer K. Culturing adult canine sensory neurons to optimise neural repair. Vet Rec. 2012; 170: 102.
- Lim JH, Koh S, Olby NJ, Piedrahita J, Mariani CL. Isolation and characterization of neural progenitor cells from adult canine brains. Am J Vet Res. 2012; 73: 1963-1968.
- Schneider MR, Wolf E, Braun J, Kolb HJ, Adler H. Canine embryo-derived stem cells and models for human diseases. Hum Mol Genet. 2008; 17: 42-47.
- Wewetzer K, Radtke C, Kocsis J, Baumgärtner W. Species-specific control of cellular proliferation and the impact of large animal models for the use of olfactory ensheathing cells and Schwann cells in spinal cord repair. Exp Neurol. 2011; 229: 80-87.
- Peruffo A, Cozzi B, Ballarin C. Ontogenesis of brain aromatase P450 expression in the bovine hypothalamus. Brain Res Bull. 2008; 75: 60-65.
- Peruffo A, Massimino ML, Ballarin C, Carmignoto G, Rota A, Cozzi B. Primary cultures from fetal bovine brain. Neuroreport. 2004; 15: 1719-1722.
- Takenouchi T, Iwamaru Y, Sato M, Yokoyama T, Shinagawa M, Kitani H. Establishment and characterization of SV40 large T antigen-immortalized cell lines derived from fetal bovine brain tissues after prolonged cryopreservation. Cell Biol Int. 2007; 31: 57-64.
- Negishi T, Ishii Y, Kawamura S, Kuroda Y, Yoshikawa Y. Cryopreservation of brain tissue for primary culture. Exp Anim. 2002; 51: 383-390.
- Cai RS, Xue DL, Jiang XH. Cryopreservation and culture of the human fetal brain tissues. J Tongji Med Univ. 1993; 13: 138-142.
- Collier TJ, Gallagher MJ, Sladek CD. Cryopreservation and storage of embryonic rat mesencephalic dopamine neurons for one year: comparison to fresh tissue in culture and neural grafts. Brain Res. 1993; 623: 249-256.
- Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985; 100: 175-188.
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990; 347: 762-765.
- Bologa L, Joubert R, Bisconte JC, Margules S, Deugnier MA, Derbin C, et al. Development of immunologically identified brain cells in culture: quantitative aspects. Exp Brain Res. 1983; 53: 163-167.
