Abstract
The culture of the nitrogen-fixing cyanobacterium Anabaena azollae, the cyanobiont of the fern Azolla, is a long-time debate. Therefore, on the present research, the cyanobiont was cultured in three culture media (AA, AA1/8, BG- 110) supplemented with an aqueous extract of Azolla filiculoides, three sugar sources (fructose, D-glucosamine, N-acetyl-glucosamine), KNO3 and B12 vitamin and their fitness was assessed by the analysis of the Phycobiliproteins (PBP) content. The results of the present research showed that the addition of a fern aqueous extract to the culture media AA, AA 1/8 and BG-110 induced the loss of A. azollae cells. Also, in all culture media without any supplements and media complemented with N-acetyl-glucosamine, KNO3, and B12 vitamin, the cyanobiont is formed by very short filaments or isolated vegetative cells and heterocysts. Regarding the PBP, the phycocyanin content almost disappeared from the cyanobiont followed by a sharp decrease in the allophycocyanin and phycoerythrocyanin in a lesser extent. The cyanobionts growing in the media AA, AA 1/8 and BG-110 without sugar source and supplements, medium AA 1/8+N-acetyl-glucosamine+B12 vitamin, and medium BG-110+N-acetylglucosamine+ B12 vitamin has phycoerythrocyaninin a higher amount than the freshly isolated cyanobiont. These changes can be due to nutritional and/or light limitations that are not adequate to this cyanobiont. Also, the culture conditions probably do not imitate the existing environment in the foliar cavities of the fern. However, N-acetyl-glucosamine seems to hinder a drastic decrease of allophycocyanin and phycoerythrocyanin, pointing to that this amino sugar can be beneficial to the growth of A. azollae.
Keywords: Anabaena azollae; Azolla filiculoides; Aqueous extract; Sugar source (fructose, D-glucosamine, N-acetyl-glucosamine); Supplements (B12 vitamin, KNO3)
Abbreviations
AA: Medium Allen and Arnon; AA 1/8: Medium Allen and Arnon at 1/8 strength; APC: Allophycocyanin; BG-110: Medium Blue-Green without Nitrogen; H-40: Medium Hoagland at 1/4 strength; KNO3: Potassium Nitrate; NaClO: Sodium Hypochlorite; PC: Phycocyanin; PEC: Phycoerythrocyanin; v/v: volume/volume
Introduction
The nitrogen-fixing cyanobacteria can form symbioses with plants in which both partners get benefits from the association. The cyanobiont has a supply of nutrients, and protection against herbivores, while the plant obtains all or almost all of the nitrogen needed for its development [1].
The fern Azolla (Figure 1A) is a unique plant because it is the only fern that has a never lasting symbiosis throughout the entire life cycle (both sexual and asexual) with a heterocystous nitrogenfixing cyanobacterium Anabaena azollae Strasburger which occupy a narrow space at the periphery of the foliar cavity leaving the centre empty (Figure 1B). The filaments of the cyanobiont contain vegetative cells, heterocysts and occasionally akinetes. The cyanobacterial cells have a Gram-negative cell wall type, inclusions in the cytoplasm (carboxysomes, cyanophycin granules, and others) and a rudimentary thylakoid system. The heterocysts are specialized cells for nitrogen fixation, with two polar nodes of cyanophycin, honeycomb-like thylakoids, thick cell wall [2] and a proteinaceous extra-sheath [3]. The high heterocyst frequency (30-40%) and high nitrogen fixation rate led several researchers to the isolation and growth of several presumptive cultivable cyanobionts from all Azolla species using different growing conditions (Table 1). The most widely used culture medium is BG-110, combined with a wide range of conditions such as light intensity (from 9 to 200 μmol/m².s), temperature (22-33°C) and supplements (different nitrogen sources, sugar sources, pH among others) turning the data comparison very difficult [4-13]. However, Tang et al. [8] using a broad array of culture conditions pointed to the difficulty in maintaining A. azollae in culture, especially due to photo bleaching and no cell multiplication. The maintenance of the green colour of the cyanobiont cells in complex culture conditions by 119 days (medium AA + 10 mM fructose + 0.05% yeast extract + 2% agar + 0.05% casamino acid + 0.47 mM NaNO3; 7 days incubation in darkness; 1% O2 and 99% N2, 10000 lux, 30°C) and 183 days (medium AA + 10 mM fructose + 0.05% casamino acids + 0.05% yeast extract + 0.35 mM NaNO3 + 2% agar; 7 days incubation in darkness; 1% O2 and 99% N2, 10000 lux, 30°C in Petri dishes) was used as a parameter to assess the viability of the cyanobiont cells [8] Compared to freshly cyanobiont isolated from Azolla cavities, the presumptive cultivable cyanobiont has low heterocyst frequency, small vegetative cells, and heterocysts, and dissimilar contents of chlorophyll a, proteins and phycobiliproteins [6-7,11-13]. The genome sequencing of A. azollae supported this difficulty due to the existence of pseudogenes or gene loss involved in the replication, repair, glycolysis and nutrient uptake [14] pointing that the presumptive cultivable cyanobiont was probably a resilient contaminant [15]. Given that the nitrogen fixation is a highly energy-demanding process and that the cyanobiont multiply inside the foliar cavities, the fern seems to be the main provider of carbohydrates for the cyanobiont [16]. But amino sugars as N-acetylglucosamine and D-glucosamine [17] can also be provided by the fern.
Growth conditions
Cyanobiont
Reference
Culture medium: BG-110
Light intensity: 5 W/m2 (9 μmol/m2.s)
Temp: 26°C
Supplements: 1) nitrate at 125, 350 ppm
2) ammonium at 10, 20, 40 ppm
3) fructose, sucrose and glucose at 2, 5, 10, 30 mM
- Nitrate at 350 ppm stimulated growth, protein and chlorophyll contents; inhibition of phycobiliprotein content and heterocyst frequency
- Ammonium at 20 ppm stimulated growth, protein, chlorophyll and phycobiliproteins contents
- Fructose (2-30 mM) stimulated nitrogen fixation
- Glucose and sucrose (2-30 mM) decreased nitrogen fixation
[4]
Culture medium: BG-110
Light intensity: 5 W/m2 (9 μmol/m2.s)
Temp: 26°C
Supplements: Fructose, sucrose and glucose at 8 mM
- Fructose stimulated higher growth than sucrose and glucose
- Fructose increased chlorophyll a and glycogen content, heterocyst frequency, nitrogen fixation and glucose-6-phosphate dehydrogenase activity
[5]
Culture medium: BG-110
Light intensity: 200 μmol/m2.s
Temp: 33°C
Supplements: 1) bubbled air and pH 9.3
2) air + 1.5% CO2, pH 6.4
3) air + 1.5% CO2 + 20 mM NaHCO3, pH 8.2
4) air + 1.5% CO2, 10 mM HEPES, pH 7.2
5) air + 1.5% CO2 + 20 mM NaHCO3, 15 mM NaNO3, pH 8.3
6) air + 1.5% CO2 + 20 mM NaHCO3, 2.5 mM NaNO3, pH 8.3
7) air + 1.5% CO2 + 20 mM NaHCO3, 5 mM NH4Cl, pH 8.0
- Aeration with CO2 and nitrogen source induced a little growth of A. azollae
- Phycocyanin increased with the addition of HEPES and ammonium
[6]
Culture medium: BG-110 (pH 7.4)
Light intensity: 135 μmol/m2.s
Temp: 22-25°C
Agitation in a water bath at 30°C
- Colonial, globular, fimbriate and tubular growth
- Vegetative cells and heterocysts size are not homogenous among cyanobiont
- Low heterocyst frequency
[7]
Growth conditions:
1) BG-110, 0.1% agar, 30°C, 10000 lux (135 μmol/m2.s), without initial darkness
2) BG-110 or AA, 30°C, 10000 lux (135 μmol/m2.s), initial 6 days in darkness
3) AA + 2 mM NH4Cl + 10 mM fructose, 4.5 days with agitation in darkness at room temperature; 3 days at 10000 lux (135 μmol/m2.s) and 30°C
4) AA + 2 mM NH4Cl + 10 mM fructose + 0.1% yeast extract (0.1% agar), pre-incubation in darkness; agitation at room temperature for 15 days; growth at 5000 lux (68 μmol/m2.s) after transfer to semi-solid media
5) AA + 5mM NH4Cl + 10 mM fructose + 0.1% agar, pre-treatment in darkness; transfer to 30°C and then increase radiation until 10000 lux (135 μmol/m2.s)
6) AA + 10 mM fructose + 0.05% yeast extract + 0.05% casamino acid + 0.47 mM NaNO3, pre-treatment in darkness; transfer to 30°C and then increase radiation until 10000 lux (135 μmol/m2.s)
7) AA + 10 mM fructose + 0.05% yeast extract + 2% agar + 0.05% casamino acid + 0.47 mM NaNO3, 7 days incubation in darkness; under 1% O2 and 99% N2, growth at 10000 lux (135 μmol/m2.s) and 30°C
8) AA + 10 mM fructose + 0.05% yeast extract + 0.05% casamino acid + antibiotic mixture + 2% agar, 7 days incubation in darkness; under 1% O2 and 99% N2, growth at 10000 lux (135 μmol/m2.s) and 30°C
9) AA + 10 mM fructose + 0.05% casamino acids + 0.05% yeast extract + 0.35 mM NaNO3 + 2% agar, 7 days incubation in darkness; under 1% O2 and 99% N2, growth at 10000 lux (135 μmol/m2.s) and 30°C in Petri dishes
- Photobleaching in cyanobiont cells
- Green color maintenance depends on culture medium, supplementation and growth conditions -highest days (119 and 183 days) in experiment 7 and 9
- Cell division only in agar cultures
[8]
Culture medium: BG-110
Light intensity: 15 μmol/m2.s
Temp: 28°C
Supplements: Fructose, glucose, sucrose, and maltose at 0.3 %
- Colonies flat and smooth
- Filaments with cylindrical vegetative cells and ellipsoidal heterocysts
- Presence of hormogonia
- Photoheterotrophic growth with fructose
- Cultured filaments did not show chromatic adaptation
[9]
Culture medium: N-free medium
Supplements: 1) NO3- and NO2- at 5 mM
2) NH4+ at 1 mM
- Cyanobionts only grown with supplements
[10]
Culture medium: BG-110
Light intensity: 52 μmol/m2.s
Temp: 25°C
Photoperiod: 16/8 h (day/night)
- Lower content of chlorophylls, phycobiliproteins, proteins, and sugars
- Low heterocyst frequency
- Filaments with small vegetative cells and heterocysts
- High nitrogen fixation
[11]
Culture medium: BG-110
Light intensity: 170 and 250 μmol/m2.s
Temp: 29°C
Photoperiod: 16/8 h (day/night)
Supplements: Glucose and sucrose at 0.5%
- Dry mass increased with high light intensity
- Protein, chlorophyll, carotenoids and phycobiliproteins content increased with high light intensity and supplements
- Nitrogen fixation increased with high light intensity
[12]
Culture medium: BG-110
Light intensity: 52 μmol/m2.s
Temp: 25°C
Photoperiod: 16/8 h (day/night)
- Low heterocyst frequency
- Coiled and straight filaments
- Small sized vegetative cells and heterocysts
- Dull green color
[13]
Table 1: Major findings of the cultivable cyanobiont A. azollae isolated from Azolla species and growth conditions.

Figure 1: Morphological aspects of A. filiculoides. A) Round sporophyte with
alternate ramifications with the chlorophyllous dorsal lobes. B) Cross-section
of the cavity (C) of the dorsal lobes with filaments of the cyanobiont A. azollae
(arrow).
The aim of the present study was to culture the cyanobiont A. azollae in three media (BG-110, AA and AA 1/8) supplemented with an aqueous extract of A. filiculoides, Potassium Nitrate (KNO3), B12 vitamin and three sugar sources (fructose, D-glucosamine, and N-acetyl-glucosamine). The cyanobiont fitness was assessed by quantification of the Phycobiliproteins Phycoerythrocyanin (PEC), Phycocyanin (PC) and Allophycocyanin (APC).
Material and Methods
Growth of Azolla filiculoides
The sporophytes of A. filiculoides (accession FI1001) obtained from the germplasm collection at International Rice Research Institute (IRRI) were grown in H-40 medium, pH 6.1-6.2, in controlled conditions [18].
Extraction of aqueous compounds from Azolla filiculoides
In a sterile flow hood, the sporophytes of A. filiculoides were washed in sterile tap water, immersed in sterile distilled water and ground with a sterile pestle. The homogenate was centrifuged at 4546 xg, for 30 min, at 4°C. The supernatant was filtered through filter paper (Whatman N° 1) to eliminate remaining debris and stored at -20°C.
Isolation of the cyanobiont Anabaena azollae
In a sterile hood, roots were cut and the rootless sporophytes were disinfected twice in an aqueous solution of sodium hypochlorite (1 ml NaClO:10 ml distilled water, v/v) for 20 min, followed by three washes in sterile ultrapure water. The cyanobiont was isolated from sporophytes of A. filiculoides using the gentle roller method described by Rai and Rai [19]. The isolated cyanobiont was inoculated in each culture medium.
Experimental design
The cyanobionts were cultured in three media - AA, AA 1/8 fold strength [20] and BG-110 [21]. The added supplements were the aqueous extract from A. filiculoides (filtered through a sterile syringe filter 0.45 μm pore diameter prior to their adding to each culture media) in1:10 (v:v, extract: culture medium), 8 mM D-(-)-fructose [5], 5 mM D-(+)-glucosamine, 5 mM N-acetyl-D-glucosamine, 1 mg/L B12 vitamin [22] and 5 mM KNO3 [9] (Figure 2). The addition of 100 mg/L of cycloheximide [23] to all the media inhibited the eukaryotic growth. The cyanobionts freshly isolated from the foliar cavities of A. filiculoides was regarded as the control. Due to a shortage of filaments of A. azollae, it was only made one replica for each medium and supplements. Following three days of darkness to prevent photobleaching [8], the cyanobionts were grown at 24°C, photoperiod of 16 h/8 h (day/night) and light intensity of 40μmol/ m²s provided by one fluorescent lamp Phillips Cool White TLD 36W. The medium was changed every 15-20 days. After 3 months, the medium was centrifuged at 4542 xg, for 5 min, at 4°C and the pellet was stored at -20°C. An aliquot of the non-frozen pellet was observed under an Olympus BX41 (Olympus, Lisbon, Portugal) light microscope.
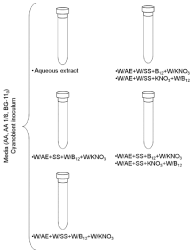
Figure 2: Diagram of the experimental design. Legend: W/AE - without
aqueous extract; W/SS - without sugar source; W/B12 - without B12
vitamin; W/KNO3 - without potassium nitrate; SS - sugar source (Fructose
or D-glucosamine or N-acetyl-D-glucosamine); B12- B12 vitamin; KNO3 -
potassium nitrate.
Extraction and quantification of phycobiliproteins
The phycobiliproteins from each assay (except the assay supplemented by an aqueous extract of A. filiculoides) and a freshly isolated A. Azollae sample were extracted following Pereira et al. [24]. The absorbance was measured at 572, 612 and 647 nm in a Synergy HT spectrophotometer (BioTek, Madrid, Spain) equipped with Gen5 2.00 software. The content of PEC, PC, and APC was calculated according to Kaplan et al. [25].
Data analysis
Prior to the analysis of variance, data of the three phycobiliproteins were transformed as a percentage to control. This data was analyzed by two-way ANOVA without replication. The significance level (a) was 0.05. Statistical analyses were made using the Microsoft® Office Excell® 2007.
Results and Discussion
One of the particularities of the Azolla-A. azollae symbiosis is the synchronous development of both partners [15] indicating that probably Azolla might have a chemical or nutritional control regarding the development, differentiation, and multiplication of the cyanobiont. In the present research and after three months in culture conditions there were no filaments or isolated cyanobacterial cells in all the three media tested to which was added an aqueous extract of A. filiculoides. Probably water-soluble compound(s) from A. filiculoides can impair the multiplication of the cyanobiont or may induce its necrosis, but nothing is known about it.
A. azollae growing in artificial conditions showed straight filaments with cylindrical vegetative cells and ellipsoidal heterocysts [7,9,11-13]. However, at the present research, in all the media (AA, AA 1/8 and BG-110) lacking any sugar source and supplements (KNO3 and B12 vitamin), the cyanobiont did not had green colour, did not grew and had very short filaments formed by cylindrical vegetative cells and ellipsoidal heterocysts with full-size inclusions (Figure 3A) or isolated vegetative cells and heterocysts (Figure 3B). The addition of a nitrate source stimulated the A. azollae growth [4,6,8,10], but in the present research, the addition of potassium nitrate as an additional nitrogen source did not induce growth of A. azollae. Moreover, since the nitrate transport gene in A. azollae is a pseudogene [13] the KNO3 is not transported to the cyanobiont cells and thus not necessary for the cyanobiont growth. Regarding the B12 vitamin, there are no studies about their use for A. azollae growth, but since after 3 months very few filaments of A. azollae were observed probably their addition is not necessary.
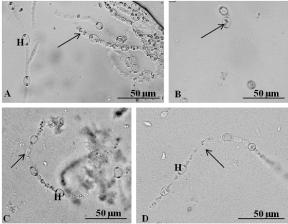
Figure 3: A. azollae growth under a light microscope. A) Filaments with
heterocysts (H) and vegetative cells (arrow) grown in BG-110 withKNO3 and
without sugar source and B12 vitamin. B) Isolated cells of the cyanobiont
(arrow) grown in medium AA without sugar source, KNO3, and B12 vitamin.
C) A short filament with heterocysts (H) and vegetative cells (arrow) of the
cyanobiont grown in medium BG-110 supplemented with fructose and KNO3
and without B12 vitamin. D) Filaments formed by vegetative cells (arrow)
and heterocysts (H) grown in medium AA supplemented with N-acetylglucosamine
and KNO3 and without B12 vitamin.
The present research showed that the addition of fructose to the medium BG-110 without KNO3 and B12 vitamin, medium BG- 110+KNO3 and medium AA+KNO3 did not induce A. azollae growth. Instead, short filaments formed by vegetative cells and heterocysts with cytoplasm inclusions and without the typical green colour were observed (Figure 3C). On the remaining combinations of culture media with fructose, the cyanobiont cells were rare and contained many bacteria. These results are in disagreement with Rosen et al. [5] and Vagnoli et al. [9], which showed that the addition of fructose increased the photo heterotrophic growth of A. azollae. This dissimilarity could be due to the different temperatures and/ or different light intensities used in the present research (40 μmol/ m².s and 24°C) and by Rosen et al. [5] (9 μmol/m².s and 26°C ) and Vagnoli et al. [9] (15 μmol/m².s and 28°C). On the other hand, Tel-Or and Sandovsky [4] showed that fructose had not induced an increase in A. azollae growth rate, which agrees with the results of the present research. In the assays made in the present research with the three media (AA, AA 1/8 and BG-110) to which was added D-glucosamine and with or without KNO3 and B12 vitamin, the cyanobiont cells were rare, did not exists filaments of the cyanobiont pointing to its almost disappearance of culture media. Thus, D-glucosamine seems to be not useful for the cyanobiont growth. However, in all the assays at the present research with the N-acetyl-glucosamine with or without KNO3 and B12 vitamin, the cyanobiont had a few short filaments formed by vegetative cells and heterocysts with inclusions and no green colour (Figure 3D), which may indicate that this amino sugar may be used as sugar source in more complex formulations of culture media.
The phycobiliproteins content can be related to chromatic adaptation or nutrient starvation such as a lower influx of carbohydrates or nitrogen [26]. In the present study, the cyanobionts growing in the medium AA with fructose and N-acetyl-glucosamine without supplements (KNO3 and B12 vitamin), N-acetyl-glucosamine + B12 vitamin and N-acetyl-glucosamine + KNO3 showed low levels of phycocyanin (Figure 4A). The most severe decrease or disappearing of phycocyanin occurred with media AA 1/8 (Figure 4B) and BG- 110 (Figure 4C), but adding N-acetyl-glucosamine appeared to hinder the total loss of phycocyanin. Probably, this may indicate that phycocyanin could be the first phycobiliprotein to be degraded or their synthesis repressed regardless medium, sugar source or other supplements added. But, the two-way ANOVA indicated that the sugar source added to the all the culture media induced statistical significant differences on the PC content (F=17.44, P<0.05) but not the type of culture media (F=0.47, P>0.05) pointing to the sugar source as an important parameter in the culture media formulation due to their possible influence on the phycocyanin content in the cyanobiont. Relating to allophycocyanin, the addition of fructose to medium AA seemed to protect against their loss from A. azollae (Figure 4D). The cyanobionts that grew in the media AA 1/8 without added sugar source and with N-acetyl-glucosamine showed a smaller loss of allophycocyanin in comparison to other growth media (Figure 4E). As to the medium BG-110, the content of allophycocyanin in A. azollae growing with N-acetyl-glucosamine without KNO3 and B12 vitamin induced a more rapid loss of allophycocyanin (Figure 4F). The analysis of variance (two-way ANOVA) showed that the sugar source added to the media induced statistical significant differences on the APC content (F=5.30, P<0.05) but not the type of culture media (F=0.80, P>0.05) again pointing to the sugar source as an important constraint in the culture media formulation and in the APC content of the cyanobiont. The content of phycoerythrocyanin surpassed the 100% in six assays - medium AA without sugar source and supplements, medium AA +N-acetyl-glucosamine + KNO3 (Figure 4G), medium AA 1/8 without sugar source and supplements, AA 1/8 + N-acetylglucosamine + B12 vitamin (Figure 4H), medium BG-110 without sugar source and supplements, BG-110 + N-acetyl-glucosamine and B12 vitamin (Figure 4I) - which means that the cultured cyanobionts have higher amounts of phycoerythrocyanin than the freshly isolates of A. azollae. The two-way ANOVA pointed to the sugar source as an important parameter in the culture media formulation and the maintenance of APC in the cyanobiont since it induced statistical significant differences on the PEC content (F=21.18, P<0.05) but not the type of culture media (F=0.83, P>0.05). The amino sugar D-glucosamine has induced almost the total loss of allophycocyanin (Figure 4D,4E,4F) and phycoerythrocyanin (Figure 4G,4H,4I) from the cyanobionts. Nitrogen starvation induced the degradation of phycobiliproteins in A. azollae [25], but Tel-Or and Sandovsky [4] showed that the phycobiliprotein content was inhibited by nitrate and Zimmerman [6] showed that phycocyanin was stimulated by the presence of ammonium. However, the phycobiliprotein data from the present research seems to indicate that the cyanobiont in culture probably suffered from a severe carbohydrate and/or nitrogen starvation. Also, the type of sugar source added to the medium and not the type of culture medium seems to have a major influence on the PBP content of the cyanobiont. Moreover, the data of the present research seems to point to that the amino sugar N-acetylglucosamine can be included in more complex formulations since it seems to hinder the total loss of phycocyanin and prevented a more drastic decrease of allophycocyanin and phycoerythrocyanin and also seems to favour the presence of cyanobiont cells but not the loss of the green colour.
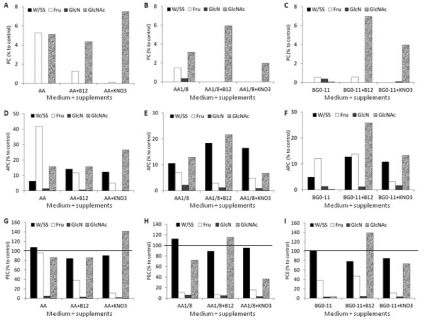
Figure 4: Phycobiliprotein content of A. azollae grown in three culture media with or without sugar source, KNO3, and B12 vitamin. Data represent the percentage
of PBP to control (fresh isolated A. azollae). A) Phycocyanin of A. azollae grown in medium AA. B) Phycocyanin of A. azollae grown in medium AA 1/8. C)
Phycocyanin of A. azollae grown in medium BG-110. D) Allophycocyanin of A. azollae grown in medium AA. E) Allophycocyanin of A. azollae grown in medium AA
1/8. F) Allophycocyanin of A. azollae grown in medium BG-110. G) Phycoerythrocyanin of A. azollae grown in medium AA. H) Phycoerythrocyanin of A. azollae
grown in medium AA 1/8. I) Phycoerythrocyanin of A. azollae grown in medium BG-110.
Conclusion
In conclusion, following 3 months in culture 1) A. azollae fade away in media with the A. filiculoides aqueous extract; 2) cyanobiont filaments became very short, did not show hormogonia, and loss the blue-green colour; 3) fructose and D-glucosamine did not benefit A. azollae growth; 4) N-acetyl-glucosamine seems to favour the maintenance of some cyanobiont filaments; 5) KNO3 and B12 vitamin are not essential for growth of A. azollae; 6) artificial growth conditions induced degradation or non-synthesis of the phycocyanin, but the allophycocyanin and phycoerythrocyanin remain in the cyanobiont; 7) the type of sugar source is more important to the PBP content than the culture media. These differences could be due to nutritional starvation and/or light intensity limitation which did not replicate the in vivo conditions inside the foliar cavities of A. filiculoides. So, knowing the metabolite exchange between the fern Azolla and the cyanobiont inside the foliar cavities should be crucial to grown A. azollae in vitro. Also, due to the high nitrogen fixation rate of A. azollae from Azolla species and other cyanobionts that form symbioses with Gunnera, cycads or lichens it is important to analyse how this is made to establish a culture in bioreactors and produce ammonium and probably a fertilizer which was not produced by chemical synthesis but by a microorganism.
Acknowledgment
Thanks to Stephan Haefele and Agnes Padre of IRRI for A. filiculoides (FI1001). The European Social Funding (FSE) under the Human Potential Operational Program (POPH) of National Strategic Reference Board (QREN) supports the fellowship SFRH/ BPD/44459/2008 to Ana L. Pereira. This research was supported by the Strategic Funding UID/Multi/04423/2013 through national funds provided by Foundation for Science and Technology (FCT) and European Regional Development Fund (ERDF), in the framework of the program PT2020.
References
- Adams DG. Symbiotic interactions. Whitton BA, Potts M, editors. In: The Ecology of Cyanobacteria. Their Diversity in Time and Space Kluwer Academic Publishers. 2000; 523-561.
- Neumüller M, Bergman B. The ultrastructure of Anabaena azollae in Azollapinnata. Physiol Plant. 1981; 51: 69-76.
- Pereira AL, Carrapiço F. An extra sheath around the heterocysts of Anabaena azollae from the aquatic macrophyte Azolla filiculoides Lamarck. Bot Lett. 2016; 163: 449-451.
- Tel-Or E, Sandovsky T. The response of the nitrogen-fixing cyanobacterium Anabaena azollae to combined nitrogen compounds and sugar. Israel J Bot. 1982; 31: 329-336.
- Rozen A, Arad H, Schönfeld M, Tel-Or E. Fructose supports glycogen accumulation, heterocysts differentiation, N2 fixation and growth of the isolated cyanobiont Anabaena azollae. Arch Microbiol. 1986; 145: 187-190.
- Zimmerman WJ. Growth, nitrogen fixation, and mass cultures of isolated Anabaena azollae. Biotechnol Lett. 1987; 9: 31-36.
- Zimmerman WJ, Rosen BH, Lumpkin TA. Enzymatic, lectin, and morphological characterization and classification of presumptive cyanobionts from Azolla Lam. New Phytol. 1989; 11: 497-503.
- Tang LF, Watanabe I, Liu CC. Limited multiplication of symbiotic cyanobacteria of Azolla spp. on artificial media. Appl Environ Microbiol. 1990; 56: 3623-3626.
- Vagnoli L, Margheri MC, Allotta G, Materassi R. Morphological and physiological properties of symbiotic cyanobacteria. New Phytol. 1992; 120: 243-249.
- Vaishampayan A, Sinha RP, Gupta AK, Häder DP. A cyanobacterial recombination study, involving an efficient N2-fixing non-heterocystous partner. Microbiol Res. 2000; 155: 137-141.
- Pabby A, Prasanna R, Nayak S, Singh PK. Physiological characterization of the cultured and freshly isolated endosymbionts from different species of Azolla. Plant Physiol Biochem. 2003; 41: 73-79.
- Venugopal V, Prasanna R, Sood A, Jaiswal P, Kaushik BD. Stimulation of pigment accumulation in Anabaena azollae strains: effect of light intensity and sugars. Folia Microbiol. 2006; 51: 50-56.
- Sood A, Prasanna R, Singh PK. Fingerprinting of freshly and cultured cyanobionts from different Azolla species using morphological and molecular markers. Aq Bot. 2008; 88: 142-147.
- Ran L, Larsson J, Vigil-Stenman T, Nylander JAA, Ininbergs K, Zheng WW, et al. Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLoS ONE. 2010; 5: e11486.
- Pereira AL, Vasconcelos V. Classification and phylogeny of the endosymbiotic cyanobacterium Anabaena azollae Strasburger: an answered question?. Int J Syst Evol Microbiol. 2014; 64: 1830-1840.
- Kaplan D, Peters GA. The Azolla-Anabaena azollae relationship. XIV. Chemical composition of the association and soluble carbohydrates of the association, endophyte-free Azolla, and the freshly isolated endophyte. Symbiosis. 1998; 24: 35-50.
- Larsson J, Nylander JAA, Bergman B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol Biol. 2011; 11: 187.
- Pereira AL, Carrapiço F. Culture of Azolla filiculoides in artificial conditions. Plant Biosyst. 2009; 143: 431-434.
- Rai AK, Rai V. Effect of NaCl on growth, nitrate uptake and reduction and nitrogenase activity of Azollapinnata-Anabaena azollae. Plant Sci. 2003; 164: 61-69.
- Allen MB, Arnon DI. Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrical Lemm. Plant Physiol. 1955; 30: 366-372.
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979; 111: 1-61.
- Waterbury JB, Watson SW, Valois FW, Franks DG. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Platt T, Li WKI, editors. In: Photosynthetic Picoplankton. Canadian Bulletin of Fisheries and Aquatic Sciences, Department of Fisheries and Oceans. 1986; 71-120.
- Gehringer MM, Pengelly JJL, Cuddy WS, Fieker C, Forster PI, Neilan BA. Host selection of symbiotic cyanobacteria in 31 species of the Australian cycad genus: Macrozamia (Zamiaceae). Mol Plant-Microbe Interact. 2010; 23: 811-822.
- Pereira AL, Monteiro B, Azevedo J, Campos A, Osório H, Vasconcelos V. Effects of the naturally-occurring contaminant microcystins on the Azolla filiculoides-Anabaena azollae symbiosis. Ecotoxicol Environ Saf. 2015; 118: 11-20.
- Kaplan D, Calvert HE, Peters GA. The Azolla-Anabaena azollae relationship. XII. Nitrogenase activity and phycobiliproteins of the endophyte as a function of leaf age and cell type. Plant Physiol. 1986; 80: 884-890.
- Grossman AR, Schaefer MR, Chiang GG, Collier JL. The responses of cyanobacteria to environmental conditions: light and nutrients. Bryant DA, editor. In: The Molecular Biology of Cyanobacteria. Academic Publishing. 1994; 641-675.
