
Mini Review
Austin Biomark Diagn. 2014;1(2): 5.
State Versus Trait Diagnostic Biomarkers in Psychiatry and the Issue of Translation
Drozdstoy St Stoyanov* and Sevdalina Kandilarova
Department of Psychiatry and Medical Psychology, Medical University of Plovdiv, Bulgaria
*Corresponding author: Drozdstoy St. Stoyanov, Medical University of Plovdiv, Bulgaria, Department of Psychiatry and Medical Psychology, Vassil Aprilov blvd 15-A, Plovdiv 4002, Bulgaria.
Received: October 17, 2014; Accepted: November 07, 2014; Published: November 14, 2014
Abstract
The trait versus state measures is highlighted as controversy in psychiatry and psychology. Trait markers are associated typically with retest stable genetic characteristics, such as personality dimensions. In clinical psychiatry trait biomarkers are incorporated into the endophenotype model. State dependent biomarkers on the other hand are case and state sensitive, appropriate for monitoring of disease course and outcome. There are reviewed Qualitative EEG (QEEG), structural and functional neuroimaging markers as related to different diagnosis and outcome from treatment in psychiatry. The issue of translation across disciplines involved in psychiatric diagnosis is considered to be underpinned by the concordance and synchronicity of the data under the model of trans-disciplinary validation.
Keywords: Traits; State dependent bio-markers; Diagnosis; Translation; Psychiatry
Trait Diagnostic Biomarkers in Psychiatry
The state versus trait controversy in determination of the diagnostic value of certain biomarkers corresponds to the same dichotomy in methodology of clinical psychology measures. Traits are defined in both settings as retest stable life time features to characterize the functioning of the system and its abnormalities The dimensions of personality are taken as typical traits in clinical psychology (e.g. temperament and character), which also have neurobiological correlates. It has been successfully demonstrated the link of traits to neurobiological processes in the psychobiological model of personality as developed by Cloninger [1,2] as well as in studies derived from Eysenck’s model of personality. In Eysenck’s theory [3] personality dimensions of extroversion is considered to be associated with the arousal of the cerebral cortex, whilst neuroticism is linked to the function of the limbic system.
In psychobiological theory of personality by Cloninger, the brain circuit regulating persistence has been determined to involve the anterior cingulate cortex (Brodmann area 24), orbitofrontal cortex (Brodmann area 47), and the ventral striatum, which regulates conditioning of reward-seeking behavior. Real-time testing of circuit activity was carried out by varying the proportion of neutral stimuli when people were asked to rate pictures as pleasant, neutral, or unpleasant during functional magnetic resonance imaging. Circuit activity increased, along with increasing proportions of neutral pictures in highly persistent people, whereas it decreased under the same conditions in impersistent people; this nonlinear effect was direct evidence of a complex adaptive system. In addition, ratings of affective valence (i.e., pleasant or unpleasant) depended on nonlinear interactions of persistence with harm avoidance and self-directedness, which themselves modulate connectivity of the anterior cingulum with the amygdala and the medial prefrontal cortex respectively [4].
In psychiatry on the other hand trait or state-independent biomarkers are typically unified under the concept of endophenotype. The term endophenotype (EP) is used often to describe a kind of trait marker that relates only to the genetically influenced characteristics of the phenotype. In the present literature there are several definitions of the EP [5] that emphasize on its heritability, association with the illness, state independence and co-segregation within families. It is expected that the EP is found at higher rate in unaffected family members than in the general population. It is assumed also that they are closer to the genes involved in the development of a disorder than are the clinical manifestations (e.g. the phenotype) and are influenced by a smaller number of genetic and environmental factors. Thus the EP approach is expected to increase the power of genetic studies of psychiatric disorders. The search for putative EPs is gaining more and more speed in the recent years and in the following lines we will try to make a short review of the most prominent findings in the area. Different endophenotype strategies have been considered for schizophrenia and bipolar disorders [5,6]. As a component of those strategies there have been performed a number of genetic and genomic studies [6,7]. However those studies need further confirmation in order to have any diagnostic validity for psychiatric nosology.
A specific approach within the endophenotype strategy of schizophrenia is the At-Risk-Mental-State paradigm [7]. It includes neuroimaging biomarkers for identifications of subjects at high risk of transition to psychosis. Structural and functional alterations in the cingulate cortex have been reported at a meta-analytical level in subjects presenting with a first episode of psychosis [8]. Meta analyses of whole brain structural studies comparing HR subjects with controls confirmed reduced gray matter volume in the HR as compared to controls in the cingulate cortices as well as in temporal, prefrontal, parahippocampal/hippocampal regions [9,10]. Volumetric reductions in cingulate and temporal, insular, prefrontal cortex and in cerebellum have been also associated with clinical outcome, the development of psychosis over follow-up [11,12].
State Dependent Biomarkers for Diagnosis and Treatment of Depression
A state-dependent marker usually is defined as characteristic of the clinical status, while a trait marker is present prior to clinical manifestation and is related to the pathophysiology of a disorder. There has been collected evidence over the past years about various state biomarkers, such as Electro-Encephalography (EEG) and brain imaging derived markers.
Potential EEG-derived markers
Quantitative EEG (QEEG) involves computerized spectral analysis of the signals, which could be much more informative in a research setting than the classical visual inspection of EEG recordings. Suggested EEG/QEEG predictors of antidepressive (AD)- treatment response include alpha and theta power, alpha asymmetry, theta cordance and ATR (antidepressant treatment response) index [14,15].
Pre-treatment (baseline) EEG markers include relatively controversial results by evaluation of Alpha power and alpha asymmetry and Theta power.
More consistent results were produced by the use of Low Resolution Electromagnetic Tomography Analysis (LORETA) [16] to measure theta activity localized specifically to the rostral Anterior Cingulate Cortex (rACC). There has been reported that rACC activity predicted treatment response with 64% sensitivity and 67% specificity. For more details please refer to Kandilarova and Stoyanov [17,18].
Treatment emergent EEG markers include Frontal Theta Cordance (FTC). Studies on frontal theta cordance present most consistent results supporting the predictive value of its early changes during AD-treatment. Cordance is derived from the absolute and relative power of the signal in different bands according to a specific formula [19]. Early change in FTC accurately predicted treatment response with 69% sensitivity, 75% specificity, 75 % positive predictive value and 69% negative predictive value.
Furthermore Iosifescu et al. [20] developed retrospectively a QEEG-derived marker called the Antidepressant Treatment Response index (ATR). It combines EEG results collected at baseline and week 1 and is presented as probability score ranging from 0 (low probability) to 100 (high probability). The ATR index predicted response to treatment with SSRIs or SNRI with 82% sensitivity, 54% specificity and 70 % overall accuracy.
The Biomarkers for Rapid Identification of Treatment Effectiveness in Major Depression (BRITE-MD) study was designed to prospectively evaluate several possible neurophysiologic and clinical measures that could be useful in AD-treatment choice [21]. It included 220 depressed subjects that received escitalopram 10 mg during the first week and were then randomly assigned to continue the same medication or switch to alternative treatment. The ATR index predicted response with 74% overall accuracy, 58% sensitivity, 91 % specificity, 88% positive predictive value, and 67% negative predictive value.
Potential neuroimaging-derived markers
Structural neuroimaging findings relevant to the treatment outcome prediction are summarized in Table 1 [22-28].
Study
Biomarker
Outcome measure
Frodl et al, Kronmuller et al. [22,23]
lower hippocampal volume
worse clinical outcome, risk of relapse
MacQueen, Yucel et al. [24]
larger pre-treatment posterior hippocampal volume
Increase of remission rate and duration
Samann, Hohn et al. [25]
larger left hippocampal volume
Beneficial treatment response
Chen, Ridler et al. [26]
greater grey matter volume in ACC
faster improvement rates, lower residual symptom scores after 8 weeks of AD treatment
Li, Lin et al. [27]
reduced grey matter volume in dorsolateral prefrontal cortex (DLPFC)
Differentiates remitters from non-remitters
Delorenzo et al. [28]
lower average fractional anisotropy (FA) in DW-MRI-derived tracts from the midbrain to the right amygdala
Non-remitters
Table 1: Structural neuroimaging findings relevant to the treatment outcome prediction are summarized in Table 1 [22-28].
Functional neuroimaging data are potentially significant especially when incorporated in multimodal assessment Figure 1, including EEG derived markers. This approach combines the special resolution of e.g. functional magnetic resonance imaging (fMRI) with the temporal resolution of the continuous record performed with QEEG. In this way simultaneous dynamics of neural correlates might be monitored in anti-depressive treatment response. There are two major groups of functional neuroimaging techniques implemented in psychiatry: positron emission tomography and fMRI Some of the most significant investigations with PET-measures are summarized in Table 2 [29-35].
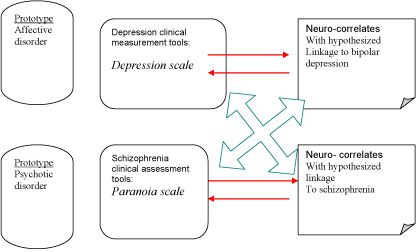
Figure 1: Multimodal diagnostic assessment in psychiatry.
Study/Authors
Biomarker
Outcome measure
Mayberg et al., Brannan et al., Pizzagalli [29-31]
Increased pre-treatment glucose metabolism in the rostral ACC
better response to various AD therapy with different methods
Brody et al. [32]
decrease in metabolism from pre- to post-treatment, ventrolateral PFC and orbitofrontal cortex (OFC)
Paroxetine responders
Drevets, Bogers et al. [33]
metabolic reduction in amygdala and right subgenual ACC
Treatment responders
Mayberg et al. [34]
decrease in activity of the limbic and striatal systems (subgenual cingulate, hippocampus, insula, and pallidum) and an increase in activity of brain stem and dorsal cortical areas (prefrontal, parietal, anterior, and posterior cingulate)
Clinical improvement
Kennedy et al. [35]
Decreased glucose metabolism bilaterally in the orbitofrontal cortex and left medial prefrontal cortex, along with increased metabolism in the right occipital-temporal cortex
Responders to Venlafaxine and Cognitive behavior therapy (CBT)
Table 2: Some of the most significant investigations with PET-measures are summarized in table 2 [29-35].
The most promising state dependent biomarkers for depression are collected with fMRI. There have been developed two major approaches: resting state and task related. Resting state fMRI has proven to produce reliable measures of functional connectivity. In a recent functional connectivity MRI study of 13 depressed patients, on various medications, Kozel et al. [36] found that treatment response correlated with the degree of connectivity between several brain regions, the most robust being the negative correlation between subcallosal cortex and the anterior cingulate cortex. In order to illustrate the potential predictive value of their finding, the authors give the following example: “choosing a connectivity value of less than 0.1 for the left subcallosal cortex to the left anterior cingulate as a predictor of treatment response, 11 of the 13 participants (85% accuracy) would have had their treatment outcome correctly ascertained prior to treatment”.
However the larger body of fMRI evidence was collected in studies using event-related or block designs where participants had to engage in different tasks. The most common task-related fMRI studies obtained sequential scans while subjects were viewing emotionally valenced (sad, happy, angry, fearful) and neutral faces.
Some of the most important findings are highlighted in Table 3 [37-47].
Study
Biomarkers
Outcome measure
Anand, Li et al., Anand, Li et al. [37,38]
Decreased corticolimbic connectivity at baseline
Lisiecka et al. [39]
higher pre-treatment connectivity of the orbitofrontal cortex (OFC); functional coupling between left OFC and the caudate nuclei and thalami
Responders
Lisiecka et al. 2011 [39]
higher OFC-cerebellum connectivity
Non-responders
Canli et al., Ruhe et al. [40,41]
Lower amygdala activation bilaterally
Responders
Samson et al. [42]
with greater pre-treatment activation in dorsomedial PFC, posterior Cingulate Cortex, and superior frontal gyrus when viewing negative emotional pictures was contrasted to the resting condition
Treatment response
Davidson et al. [43]
greater relative anterior cingulate activation at baseline in response to negative versus neutral stimuli
Treatment response
Keedwell et al. [44]
right visual cortex and right subgenual cingulate during the presentation of sad stimuli
good clinical outcome
Delaveau et al. [45]
normalization of the hypoactivity of neocortical areas and the hyperactivity of limbic and paralimbic areas
Treatment response
Fu et al. [46]
increased activation in Anterior CC and medial prefrontal cortex
higher response rate
Fu et al. [47]
increased activation in right putamen and right anterior insula; decreased right hippocampal volume
poor response to treatment
Table 3: Some of the most important findings are highlighted in Table 3 [37-47].
Instead a Conclusion: The Issue of Translation
The emergent issue of trans-disciplinary validation or translation is defined as ability of the different databases involved in psychiatry to be translated one to another in order to underpin sound explanatory models and diagnostic strategies [48-51].
Contrastingly to other medical disciplines, where inter-disciplinary translation is an established standard, in clinical psychiatry it is yet far out of reach. In our model of translation we aim at integration of the diagnostic biomarkers from in-vivo neuroimaging by real time application of clinical assessment tools, QEEG and fMRI. In this way it is provided concordance and synchronization of the measures, which is an utmost prerequisite for establishment of sound validity across disciplines involved in psychiatric diagnosis.
As it is illustrated the translational validity across disciplines is constituted by means of both divergent (blue arrows) and convergent (red arrows) validation of clinical assessment tools with in-vivo bio-markers of diagnosis in real time [52]. The translation is considered as correspondence (concordance) between the domains of expertise.
References
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993; 50: 975-990.
- Cloninger R. Degeneracy of categorical disease paradigms. Philosophy, Psychiatry & Psychology. 2013; 20: 275-279.
- Eysenck HJ, Eysenck SBG. Personality Structure and Measurement. London: Routledge. 1969.
- Stoyanov D, Machamer P, Schafner K. A Fallacious Forced Choice: Cloninger and Stoyanov, Machamer and Schafner are compatible. Phylosophy, Psychiatry & Psychology. 2013; 20: 281-284.
- Akabaliev V, Arabadjiev Z, Akabalieva K, Kandilarova S. Potential risk biomarkers in psychopathology: an update and critical reappraisal. In: Stoyanov D, editor. Psychopathology: theory, perspectives and future approaches. New York Nova Science Publishers. 2012; 253-282.
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006; 60: 93-105.
- Yosifova A, Mushiroda T, Stoianov D, Vazharova R, Dimova I, Karachanak S, et al. Case-control association study of 65 candidate genes revealed a possible association of a SNP of HTR5A to be a factor susceptible to bipolar disease in Bulgarian population. J Affect Disord. 2009; 117: 87-97.
- Betcheva ET, Yosifova AG, Mushiroda T, Kubo M, Takahashi A, Karachanak SK, et al. Whole-genome-wide association study in the Bulgarian population reveals HHAT as schizophrenia susceptibility gene. Psychiatr Genet. 2013; 23: 11-19.
- Stoyanov D, Borgvardt S, Varga S. The problem of translational validity across neuroscience and psychiatry. In: Zachar P, Stoyanov D, Aragona M, Jablenski A, editors. Alternative perspectives on psychiatric validation. Oxford: Oxford University Press. 2014; 128.
- Koutsouleris N, Borgwardt S, Meisenzahl EM, Bottlender R, Moller HJ, Riecher-Rössler A. Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: results from the FePsy study. Schizophr Bull. 2012; 38: 1234-1246.
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011; 35: 1175-1185.
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011; 127: 46-57.
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010; 34: 1207-1222.
- Hunter AM, Cook IA, Leuchter AF. The promise of the quantitative electroencephalogram as a predictor of antidepressant treatment outcomes in major depressive disorder. Psychiatr Clin North Am. 2007; 30: 105-124.
- Iosifescu DV. Electroencephalography-derived biomarkers of antidepressant response. Harv Rev Psychiatry. 2011; 19: 144-154.
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994; 18: 49-65.
- Kandilarova S, Stoyanov D. Clinical application of the theory of translational validation: potential EEG- and fMRI-derived markers of antidepressant treatment response. Folia Medica. 2014; 56: 66-67.
- Kandilarova S, Stoyanov D. Clinical application of the theory of translational validation: potential EEG- and fMRI-derived markers of antidepressant treatment response. In: Stoyanov D, editor. Towards new philosophy and mental health: perspectives form neuroscience and humanities. Forthcoming. New Castle: Cambridge Scholars Publishing. 2014.
- Leuchter AF, Cook IA, Lufkin RB, Dunkin J, Newton TF, Cummings JL, et al. Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994; 1: 208-219.
- Iosifescu DV, Greenwald S, Devlin P, Mischoulon D, Denninger JW, Alpert JE, et al. Frontal EEG predictors of treatment outcome in major depressive disorder. Eur Neuropsychopharmacol. 2009; 19: 772-777.
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Burgoyne KS, Howland RH, et al. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE-MD study. Psychiatry Res. 2009; 169: 124-131.
- Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008; 33: 423-430.
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, et al. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008; 192: 472-473.
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008; 64: 880-883.
- Sämann PG, Höhn D, Chechko N, Kloiber S, Lucae S, Ising M, et al. Prediction of antidepressant treatment response from gray matter volume across diagnostic categories. Eur Neuropsychopharmacol. 2013; 23: 1503-1515.
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry. 2007; 62: 407-414.
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010; 50: 347-356.
- Delorenzo C, Delaparte L, Thapa-Chhetry B, Miller JM, Mann JJ, Parsey RV. Prediction of selective serotonin reuptake inhibitor response using diffusion-weighted MRI. Front Psychiatry. 2013; 4: 5.
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997; 8: 1057-1061.
- Brannan SK, Mayberg HS, McGinnis S, Silva JA, Tekell J, Mahurin RK, et al. 355. Cingulate metabolism predicts treatment response: a replication. Biological Psychiatry. 2000; 47: S107.
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011; 36: 183-206.
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999; 91: 127-139.
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002; 12: 527-544.
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000; 48: 830-843.
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007; 164: 778-788.
- Kozel FA, Rao U, Lu H, Nakonezny PA, Grannemann B, McGregor T, et al. Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Front Psychiatry. 2011; 2: 7.
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005; 30: 1334-1344.
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007; 19: 274-282.
- Lisiecka D, Meisenzahl E, Scheuerecker J, Schoepf V, Whitty P, Chaney A, Moeller HJ. Neural correlates of treatment outcome in major depression. Int J Neuropsychopharmacol. 2011; 14: 521-534.
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005; 16: 1267-1270.
- Ruhe HG, Booij J, Veltman DJ, Michel MC, Schene AH. Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: a functional magnetic resonance imaging study. J Clin Psychiatry. 2012; 73: 451-459.
- Samson AC, Meisenzahl E, Scheuerecker J, Rose E, Schoepf V, Wiesmann M, et al. Brain activation predicts treatment improvement in patients with major depressive disorder. J Psychiatr Res. 2011; 45: 1214-1222.
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003; 160: 64-75.
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010; 120: 120-125.
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011; 130: 66-74.
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004; 61: 877-889.
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013; 52: 75-83.
- Zachar P, Stoyanov D, Aragona M, Jablenski A. Alternative perspectives on psychiatric validation. Oxford: Oxford University Press. 2014.
- Stoyanov D, Stieglitz R, Borgwardt S. The translational validation as novel approach to integration of neuroscience and psychiatry. In: Mishara A, Corlett P, Fletcher P, editors. Phenomenological Neuropsychiatry: how patient experience bridges clinic and clinical neuroscience. New York: Springer Science.
- Stoyanov D, Stanghellini G, Broome M. Conceptual issues in psychiatric neuroimaging: an update. Curr Top Med Chem. 2012; 12: 2348-2356.
- Stoyanov D. A linkage of mind and brain: towards translational validity between neurobiology and psychiatry. Biomedical Reviews. 2011; 22.
- Stoyanov D, Machamer P, Schaffner KF. In quest for scientific psychiatry: Toward bridging the explanatory gap. Philosophy, Psychiatry & Psychology. 2013; 20: 261-273.