
Case Report
Austin Biomark Diagn. 2015;2(1): 1017.
Distinct Immunophenotypic Features of Ovarian Microcystic Stromal Tumor
Niu S and Peng Y*
Department of Pathology, University of Texas Southwestern Medical Center, USA
*Corresponding author: Peng Y, Department of pathology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390- 9072, USA,
Received: April 09, 2015; Accepted: May 25, 2015; Published: June 04, 2015
Abstract
Ovarian Microcystic Stromal Tumor (MCST) is a newly recognized entity with only 19 reported cases, in which only 3 cases showed a solid growth pattern and 1 with concurrent endometrial carcinoma. Recent studies suggested the involvement of the Wnt/β-catenin pathway in MCST tumorigenesis through mutated β-catenin. Here we report another MCST case with a solid growth pattern, distinct immunophenotypes including β-catenin and cyclin D1 (a known β-catenin regulated oncogene), and a coexisting endometrial carcinoma. Macroscopically, the tumor was a well-circumscribed, solid mass. Microscopically, it consisted of predominantly lobulated cellular nodules separated by hyalinized fibrous stroma. Immunophenotypically, the tumor was diffusely and strongly positive for CD10, β-catenin (nuclear) and cyclin D1, and completely negative for inhibin, calretinin, ER, PR, cytokeratins, EMA and c-Myc. To our knowledge, this is the first report to demonstrate the co-over expression of β-catenin and cyclin D1 in case with MCST and concurrent endometrial carcinoma, which may provide new insights to the tumor biology.
Keywords: Microcystic stromal tumor; Ovary; β-catenin; Cyclin D1; Endometrial carcinoma
Introduction
Ovarian Microcystic Stromal Tumor (MCST) is a newly recognized entity with only 19 reported cases [1-3]. Of the 19 cases, 7 cases with follow-up data (1.5 to 12 years) revealed that the tumors had no metastasis or recurrence [1]. Grossly, MCST tumors were solid-cystic (14/19), solid (3/19), or predominantly cystic (2/19) [1-3]. Microscopically, the neoplasms were characterized by a microcystic growth pattern with variably solid cellular areas separated by fibrous stroma. The characteristic immunophenotypes was CD10+/vimentin+/inhibin-/calretinin-/EMA-(19/19). Two recent studies demonstrated diffuse and strong β-catenin protein nuclear expression in cases with MCST (3/3) [2,3]. Meanwhile a point mutation, CTNNB1 S33C, was identified (2/2), and the authors proposed a potential involvement of the Wnt/β-catenin pathway in MCST tumorigenesis [2].
Here we report another MCST case with a pure solid growth pattern grossly and microscopically, and with a distinct immunophenotypes including cyclin D1 (diffusely positive) and c-Myc (completely negative). Both cyclin D1 and c-Myc are wellestablished oncogenes involved in various tumor developments and have been shown to be direct Wnt/β-catenin targets [4-6]. Therefore, our findings may provide new insights to the MCST tumorigenesis.
Case Report
The patient is a 42-year-old obese woman (BMI 33.7) with a life-long history of heavy, irregular menses. An endometrial biopsy was performed; an endometrioid adenocarcinoma was diagnosed. On pre-surgery imaging work up, a 4.5-cm left ovarian mass was incidentally found. The patient underwent a total hysterectomy and bilateral salpingo-oophorectomy procedure. The specimens revealed FIGO grade I endometrioid adenocarcinoma with minimal myometrial invasion and focal cervical stromal involvement as well as a microcystic stromal tumor of the left ovary. The right ovary and bilateral fallopian tubes were unremarkable.
Tissue manipulation
All the tissue samples were fixed in formalin and embedded in paraffin. Full tissue sections were used for immunohistochemistry. Immunohistochemical staining was performed on an automated immunostainer (Dakoautostainer, Carpentaria, CA). Appropriate positive and negative controls were included. A list of antibodies used in this case is as follows: CD10 (Clone 56C6, Predilute);Cyclin D1 (Clone EP12, Predilute);Inhibin (Clone R1, Predilute);Calretinin (Clone DAK Calret1, 1:100); Cytokeratin (Clone AE1/AE3, Predilute); EMA (Clone E29, Predilute);Vimentin (Clone V9, 1:200); WT1 (Clone 6F-H2, Predilute); Estrogen receptor (Clone EP1, Predilute); Progesterone receptor (Clone PgR636, 1:350); Melan A (Clone A103, Predilute); and CD56 (Clone 123C3, 1:100). The above antibodies were from DAKO North America (Carpinteria, CA).C-Myc (Clone EP121, Predilute, Cell Marque, Rocklin, CA); CK14 (Clone LL002, Predilute, Cell Marque, Rocklin, CA); β-catenin (Clone 14, 1:1600, BD Biosciences, SanJose, CA).
Pathologic Findings
Macroscopically, the tumor was well circumscribed and had a white-tan, solid and nodular appearance (Figure 1A). It was confined to the left ovary. The tumor was extensively sampled and multiple tissue blocks were submitted for histologic evaluation. Microscopically, at low power of view, the tumor consisted of predominantly lobulated, cellular nodules separated by hyalinized fibrous stroma (Figure 1B). A rim of unremarkable ovarian parenchyma was present at the periphery of the lesion (Figure 1C). The lesion showed a pure solid growth pattern and no microcystic formation (Figure 1D). Rare foci of the tumor cells had abundant intracellular vacuoles (Figure 1E). At high magnification, the tumor cells were uniform and had round nuclei, open chromatin, inconspicuous nucleoli, and focally clear cytoplasm (Figure 1F). The vast majority of the tumor cells showed no nuclear grooves and nuclear membrane irregularity; only occasional nuclear grooves were appreciated. Mitotic count was up to 3 mitoses/10HPFs.
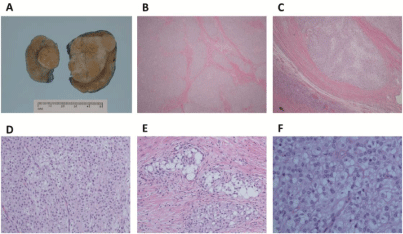
Figure 1: Pathologic features of the MCST. A, Grossly, the tumor was well circumscribed and confined to the ovary; it had a white-tan, solid and nodular
appearance. B & C, Microscopically, at lower power view, the tumor consists of predominantly lobulated cellular nodules separated by hyalinized fibrous stroma. A
rim of unremarkable ovarian parenchyma was present at the periphery of the lesion. D, The tumor cells with a solid growth pattern. E & F, at high power view, the
uniform tumor cells with round nuclei, open chromatin, inconspicuous nucleoli and intracytoplasmic vacuoles.
Immunophenotypically, the tumor was diffusely and strongly positive for Vimentin, CD10 (Figures 2A and 2B) and WT-1 (nuclear staining), while the background non-neoplasmic ovarian parenchyma was negative for these markers as described previously [1-3]. The tumor was completely negative for inhibin and calretinin (Figures 2C and 2D), Melan A, Estrogen Receptor (ER) and Progesterone Receptor (PR) (Figures 2E and 2F), cytokeratin AE1/AE3, EMA, CK14 and CD56. Proliferative index, Ki-67 (MIB-1) was less than 5%. Recent studies suggested the involvement of the Wnt/β-catenin pathway in MCST tumorigenesis through mutated β-catenin [2,3]. Both cyclin D1 and c-Myc are oncogenes that are direct targets of nuclear β-catenin [4-6]. To examine protein expression status of these biomarkers in MCST, we performed immunohistochemistry staining for β-catenin, cyclin D1 and c-Myc. β-catenin stain showed strong and diffuse nuclear staining in the neoplastic cells (Figure 2G), which was consistent with the previous reports [2,3]. Cyclin D1 also showed strong and diffuse nuclear staining (Figure 2H), while c-Myc was completely negative throughout the tumor (Figure 2 I); these findings have not been reported on the literature.
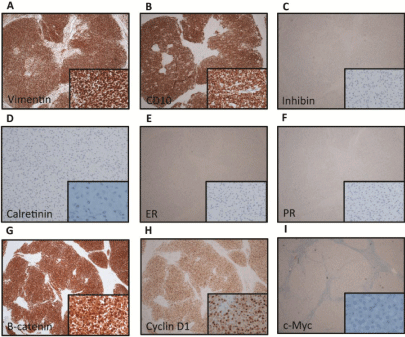
Figure 2: Immunohistochemistry profile of the MCST (Insets are high power views 20X): A & B, the tumor was diffusely and strongly positive for Vimentin and
CD10, while the non-neoplasmic ovarian stroma was negative for these markers. C & D, The tumor was completely negative for inhibin and calretinin. E & F, The
tumor was negative for ER and PR. G & H, The tumor showed strong and diffuse nuclear staining for β-catenin and cyclin D1. I, the tumor was negative for c-Myc.
Discussion
Among 19 reported MCST cases [1-3], the patients were 26-63 years of age, with a mean of 44. The tumor size ranged from 2.0 to 27.0 cm. The tumors were unilateral in all cases. There was no evidence of recurrence or extra ovarian spread, even though the data are limited. Therefore, MCST is considered benign or with low malignant potential. In the current case, MCST was unilateral and confined to the ovary. It showed a solid growth pattern not only on gross examination but also on microscopic evaluation. Its morphology is compatible with histologic features of MCSTs previously reported. Immunophenotypically, the tumor showed a typical profile of MCST, CD10+/vimentin+/inhibin-/calretinin-/CK (AE1/AE3)-/EMA- and low Ki67 expression less than 5%. Among the 19 reported cases, only 5 of 19 (26%) were CK AE1/AE3 positive and 1 of 19 (5%) inhibin positive [1-3]. All of the 19 cases (100%) had Ki67 expression less than 10% [1-3].
In addition, we performed β-catenin, as well as cyclin D1 and c-Myc staining in light of findings of a recent study [2], which showed strong nuclear β-catenin immunoreactivity in two cases with MCST and identified a point mutation in β-catenin gene. This mutation can cause deregulation of β-TrCP-mediated β-catenin degradation, which subsequently led to the nuclear accumulation of β-catenin and therefore aberrant activation of the Wnt/β-catenin signaling pathway [7]. Wnt/β-catenin plays important roles in embryonic development and self-renewal in adult tissues, and the dysregulation of this pathway has been well documented to contribute to the tumorigenesis of a wide variety of tumors. Both Cyclin D1 and c-Myc are well-known β-catenin regulated oncogenes [4-6]. The co expression of β-cateninand cyclin D1 proteins has been reported in various malignancies, and dysregulation of cyclin D1 due to β-catenin aberrant nuclear accumulation has been proposed to promote tumorigenesis in these cancers [8-13]. Our demonstration of the co-expression of cyclin D1 protein with β-catenin protein in MCST provides further evidence to support the involvement of Wnt/ β-catenin in the tumor biology of MCST.
The immunoprofile of MCSTs in the current case and the 19 previously reported cases is summarized in Table 1.
Table 1: Immunoprofile of MCSTs in the current case and all 19 previously reported cases.
Another interesting point in our case is that the patient had a concurrent FIGO grade I endometrial endometrioid adenocarcinoma. Taking together with another previously reported case [1], patients with MCST have a 10% of chance to have an endometrial endometrioid adenocarcinoma (2/20). Interestingly, simultaneous nuclear accumulations of β-cateninand cyclin D1 proteins have also been reported in endometrial endometrioid adenocarcinoma, and were proposed to play a role in type I endometrial carcinogenesis [11,12]. Therefore, in our opinion, these two tumors seen in our case might be related through the activation of the β-cateninand cyclin D1 pathway rather than estrogen status. To our knowledge, the current case is the first report to demonstrate cyclin D1 protein co-expression with β-catenin protein in MCST with a concurrent endometrial endometrioid adenocarcinoma. This might provide new insights into the tumorigenesis of this rare entity. It is noted that ER and PR immunostains performed in a total of three MCST cases including the current one were negative (3/3) [2].
In contrast to the co expression of β-cateninand cyclin D1, c-Myc that is another downstream target of β-catenin, stained completely negative in our MCST case, suggesting that aberrant signal of the pathway is more delicately regulated than the simple accumulation of a normally functioning β-catenin in the nuclei. Interestingly, a decreased c-Myc expression was also reported in colorectal cancer cases with β-catenin and cyclin D1 coexpresion [10].
A stromal nature of MCSTs is favored because of the morphologic resemblance to ovarian the coma [1-3]. In addition, a recent report provided ultra structural evidence for a stromal origin of the tumor [3]. The main differential diagnosis includes but not limited to ovarian the coma and Adult Type Granulosa Cell Tumors (AGCTs). Typical histological features of AGCT include coffee bean shaped nuclei with nuclear grooves and the presence of Call-Exner bodies that are microacini containing luminal eosinophilic secretions. However, some of AGCTs show a solid growth pattern and have only rare nuclear grooves and no Call-Exner bodies; those could morphologically resemble solid type MCSTs. As previous studies demonstrated, AGCTs are considered as low grade malignancies with recurrence even after many years of tumor removal by surgery [14]. Therefore differentiating it from MCST is crucial for prognosis and management. Concurrent endometrial endometrioid carcinoma is not uncommonly seen in AGCTs due to the high estrogen status [14], which may be different from a mechanism led to the coexistence of the endometrial carcinoma and MCST. Based on reported immunostain results on MCSTs and our results, we recommend using an immunohistochemistry panel including CD10, inhibin, calretinin, ER, PR, β-catenin and cyclin D1 to distinguish MCST from AGCT or the coma when morphologic features on those ovarian tumors are overlapping or not classic. The coma and AGCT are usually inhibin and calretinin strongly and diffusely positive, ER and PR positive, and CD10 negative or weakly and focally positive [15]. In contrast, MCSTs are commonly inhibin and calretinin negative, ER and PR negative, but with diffuse and strong positivity for CD10.
In summary, our case demonstrated that not only β-catenin but also its downstream direct target, cyclin D1 showed diffuse and strong immunoreactivity in the nuclei of MCST tumor cells. This is the second MCST case with a concurrent low-grade endometrial endometrioid adenocarcinoma, which has also been shown, for the first time, to have co-over expression of β-catenin and cyclin D1. It further supports the proposed role of Wnt/β-catenin signaling pathway in and provides new insights to the tumorigenesis of MCST. Meanwhile, this is also the 4th MCST case that showed a complete solid growth pattern. In our opinion, our results extended the knowledge on MCST of the ovary, a recently recognized rare entity.
The abstract was presented at ASCP annual meeting, October 8-10, 2014, Tampa, Florida.
References
- Irving JA, Young RH. Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm. Am J Surg Pathol. 2009; 33: 367-375.
- Maeda D, Shibahara J, Sakuma T. β-catenin (CTNNB1) S33C mutation in ovarian microcystic stromal tumors. Am J Surg Pathol. 2011; 35: 1429-1440.
- Yang M, Bhattacharjee MB. Ovarian microcystic stromal tumor: report of a new entity with immunohistochemical and ultrastructural studies. Ultrastruct Pathol. 2014; 38: 261-267.
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006; 127: 469-480.
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011; 11: 558-572.
- Wang C, Lisanti MP, Liao DJ. Reviewing once more the c-myc and Ras collaboration: converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle. 2011; 10: 57-67.
- Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002; 160: 1361-1369.
- Saitoh G, Sugio K, Ishida T, Sugimachi K. Prognostic significance of p21waf1, cyclin D1 and retinoblastoma expression detected by immunohistochemistry in non-small cell lung cancer. Oncol Rep. 2001; 8: 737-743.
- Ueta T, Ikeguchi M, Hirooka Y, Kaibara N, Terada T. β-catenin and cyclin D1 expression in human hepatocellular carcinoma. Oncol Rep. 2002; 9: 1197-1203.
- Wang HL, Wang J, Xiao SY, Haydon R, Stoiber D, He TC, et al. Elevated protein expression of cyclin D1 and Fra-1 but decreased expression of c-Myc in human colorectal adenocarcinomas over expressing beta-catenin. Int J Cancer. 2002; 101: 301-310.
- Narita F, Sato A, Hamana S, Deguchi M, Otani T, Maruo T. Simultaneous immunohistochemical localization of beta-catenin and cyclin D1 in differentiated but not in undifferentiated human endometrial carcinoma. Eur J Gynaecol Oncol. 2003; 24: 129-134.
- Shih HC, Shiozawa T, Miyamoto T, Uchikawa J, Feng Y, Kashima H, et al. Nuclear localization of beta-catenin is correlated with the expression of cyclin D1 in endometrial carcinomas. Anticancer Res. 2003; 23: 3749-3754.
- Lantsov D, Meirmanov S, Nakashima M, Kondo H, Saenko V, Naruke Y, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. Histopathology. 2005; 47: 248-256.
- Koukourakis GV, Kouloulias VE, Koukourakis MJ, Zacharias GA, Papadimitriou C, Mystakidou K, et al. Granulosa cell tumor of the ovary: tumor review. Integr Cancer Ther. 2008; 7: 204-215.
- Oliva E, Garcia-Miralles N, Vu Q, Young RH. CD10 expression in pure stromal and sex cord-stromal tumors of the ovary: an immunohistochemical analysis of 101 cases. Int J Gynecol Pathol. 2007; 26: 359-367.