
Research Article
Austin Biomark Diagn. 2015;2(2): 1018.
Red Blood Cell Distribution Width as a Predictor of Pulmonary Valve Replacement in Patients with Repaired Tetralogy of Fallot
Weinreich MA¹ and Ephrem G²*
¹Department of Medicine, Hofstra North Shore-LIJ School of Medicine, USA
²Department of Cardiovascular Disease, Oakland University-William Beaumont School of Medicine, USA
*Corresponding author: Ephrem G, Department of Cardiovascular Disease, Oakland University-William Beaumont School of Medicine, 3601 West 13 Mile Road, Royal Oak, MI 48073, USA
Received: July 13, 2015; Accepted: August 17, 2015; Published: August 25, 2015
Abstract
Background: The timing of pulmonary valve replacement (PVR) in patients with repaired Tetralogy of Fallot (rToF) is an important factor in adult congenital heart disease care. High red blood cell distribution width (RDW) is an independent predictor for recourse to cardiac surgery. This study assesses the relation between RDW and cardiac magnetic resonance imaging (cMRI) in predicting recourse to PVR.
Methods: The study is a retrospective observational cohort analysis. Data were gathered by review of electronic medical records. The relation between a high RDW and PVR was assessed, with a focus on comparing the area under the receiver operating characteristics (AUC) with those of the cMRI-based right ventricle (RV) measurements: RV end diastolic volume index (RVEDVI) >150 ml/m2, RV end systolic volume Index (RVESVI) >85 ml/m2, and RV ejection fraction (RVEF) <45%.
Results: In 44 rToF patients (26 PVR), the cMRI-RV measurements did not show any statistically significant association with PVR (RVEDVI OR 1.04, p=0.979; RVESVI OR 1.62, p=0.765; RVEF OR 0.64, p=0.573 respectively). An RDW=15% was associated with PVR (OR 2.05) but did not reach statistical significance (p=0.559). The AUCs showed similar findings. There was no statistically significant difference between the C-statistics (p=0.868).
Conclusion: In a sample population of adult rToF patients, there was no statistically significant difference between a high RDW and cMRI-based RV measurements in predicting PVR. An inexpensive and readily available marker, RDW warrants further investigation in large multicenter datasets to fully determine its role as an additional predictive biomarker for PVR in adult ToF patients.
Keywords: Tetralogy of fallot; Red cell distribution width; Pulmonary valve replacement
Abbreviations
PVR: Pulmonary Valve Replacement; ToF: Tetralogy of Fallot; rToF: Repaired ToF; RDW: Red Cell Distribution Width; cMRI: Cardiac Magnetic Resonance Imaging; RV: Right Ventricle; AUC: Area Under the Receiver Operative Characteristics; RVEDVI: RV End Diastolic Volume Index; RVESVI: RV End Systolic Volume Index; RVEF: RV Ejection Fraction; OR: Odds Ratio; MI: Myocardial Infarction
Background
Tetralogy of Fallot (ToF) is the most common congenital cyanotic heart disease. The syndrome is characterized by the compilation of ventricular septal defect, right ventricular hypertrophy, an overriding aorta, and pulmonary artery obstruction. The pathology is noted in roughly 1 in 3,600 live births and almost always requires surgical intervention [1]. Techniques of repair have evolved with time, but the mainstay of treatment remains patching of the ventricular septal defect and opening the pulmonary artery outflow tract. With cutting edge improvements in therapy, 85% of children with ToF are likely to reach adulthood and 90% have a 40-year survival rate. Despite these successes, many patients experience sequelae later in life, namely congestive heart failure, arrhythmias, or sudden cardiac death [1,2]. The etiology of these symptoms is believed to be secondary to chronic pulmonary valve insufficiency. Many patients require eventual pulmonary valve replacement (PVR) to alleviate the associated comorbidities. While PVR is imperative for this goal, the timing of intervention remains controversial.
Currently, the gold standard to determine the timing for PVR remains based on assessment of right ventricular status as measured by cardiac magnetic resonance imaging (cMRI). Imaging is recommended every 2-3 years to monitor both right ventricular (RV) volume and systolic function. The American College of Cardiology/American Heart Association 2008 Guidelines for the Management of Adults with Congenital Heart Disease provides Class IIa recommendations for PVR in rToF adults with severe pulmonary regurgitation with either moderate to severe RV dysfunction, moderate to severe RV enlargement, symptomatic arrhythmias, or moderate to severe tricuspid regurgitation [3]. However serial cMRIs are both costly and time consuming. They are also contraindicated in patients with implanted cardiac defibrillators. Thus, other predictive modalities are being explored.
Red blood cell distribution width (RDW) is a hematologic measurement of the variation in erythrocyte diameter. Elevated RDW has been reported as a marker of oxidative stress, dysregulated erythropoiesis, and anemia [4]. As patients with repaired ToF age, worsening pulmonary valve regurgitation causes remodeling of the right ventricle with resultant congestive heart failure and poor oxygenation. This hypoxemic state will drive hematopoiesis and contribute to elevations in the RDW. Recent reports have found RDW a valuable prognostic biomarker in patients with heart failure [5,6], pulmonary hypertension [7], myocardial infarction [8,9], coronary artery disease (CAD) [10,11], or in those undergoing angiography [12,13]. Other studies suggest its value in predicting readmission [14], recourse to cardiac surgery [15], and postoperative recovery after ToF repair [16]. The present study examines the relation between a high RDW and PVR, with a focus on comparing its area under the receiver operating characteristic (AUC) with those of the cMRI-based RV measurements. The hypothesis is that high RDW is a readily available and relatively inexpensive predictor for recourse to PVR in rToF patients.
Methods
Study population
The present retrospective, observational, cohort study was conducted at North Shore University Hospital and Long Island Jewish Medical Center, two tertiary care centers of the North Shore-Long Island Jewish Health System in New York. The patient population included all patients age 18 years or older with rToF who were seen at the adult congenital heart disease (ACHD) clinic between January 1, 2010 and August 31, 2014.
Data collection
Laboratory and imaging data was obtained via electronic chart review. Data regarding age, gender, body mass index, age at ToF repair, common laboratory studies (including RDW), and cMRI findings were recorded. Longitudinal follow-up ended on February 1, 2015, and the outcomes (PVR) were recorded from the electronic databases.
Definitions
Three imaging parameters of right ventricular size and function were utilized and compared based on standardized cutoff values for optimal outcomes [17]: RV end diastolic volume index (RVEDVI) >150 ml/m2, RV end systolic volume index (RVESVI) >85 ml/m2, and RV ejection fraction (RVEF) <45%. The RDW level (along with complete blood count results) had to be obtained 6 months or more prior to PVR or end of follow up if no intervention was performed. The rationale was to avoid perioperative fluctuations or acute changes at the time of censoring. RDW =15% was classified as an elevated value based on previous literature [18,19] and on the calibration and the reported normal range of the health system’s laboratory. Of note, decision for valve replacement was made at the treating physician’s discretion, likely through a combination of cMRI results, clinical status, and presence of co-morbidities.
Ethics
The study was approved by the North Shore-Long Island Jewish Health System Institutional Review Board. The requirement for obtaining an informed consent was waived. There were no conflicts of interest.
Statistical analysis/Calculation
Data were analyzed using Stata version 11.2 (StataCorp, College Station, TX). Categorical data were analyzed using Chi square or Fisher exact tests. Continuous variables whose distribution followed the normality assumptions were analyzed using the Student t test. Variables whose distribution did not approximate normality were analyzed using the nonparametric Wilcoxon rank sum tests. Multivariable logistic regression analyses were used to determine the independent relation of an elevated RDW as well as the cMRI-based RV measurements and PVR. A receiver operating characteristic (ROC) analysis was performed to assess the sensitivity of these prediction variables. The multivariate models were adjusted for all the variables that were of statistical significance in the bivariate analyses. Assessment for confounders and effect modifiers was performed and none were detected. Regression diagnostics and assessment of the fit of the models were conducted via the Hosmer-Lemeshow goodness of fit test, which showed that they fit well. All tests were 2-tailed, and P values <0.05 were considered statistically significant.
Results
Among the 44 rToF patients who were seen at the institution’s ACHD clinic, 26 had PVR. The baseline characteristics of the study subjects are listed in Table 1. On average the patients were 30 years old, almost evenly split across the gender divide, and had their ToF repaired around 3 years of age. They were not anemic and had good left and right ventricular sizes and functions. The bivariate analyses by outcome (PVR) showed no statistically significant differences in characteristics except for hemoglobin and hematocrit which were lower in the PVR group, likely from post-operative complications. This significant finding was not coupled with a statistically significant difference in RDW levels (13.5% versus 13.3%, p=0.691). The cMRIRV measurements did not show any statistically significant differences between the 2 groups either (Table 1).
Overall (n = 44)
No PVR (n = 18)
PVR (n = 26)
p
Age (y)
29.75±9.10
31.83±10.15
28.31±8.20
0.210
Male
20 (45%)
6 (33%)
14 (54%)
0.179
Age at repair (months)
36 (43)
36 (52)
36 (23)
0.510
Body mass index (kg/m2)
25.25 (8.20)
26.15 (60)
23.65 (10.30)
0.327
WBC (1,000 per count)
7.70 (2.40)
7.90 (1.80)
7.65 (2.50)
0.703
Hemoglobin (g/dL)
13.60 (2.10)
13.20 (2.10)
13.75 (2.00)
0.308
Hematocrit (%)
40.10 (5.60)
38.40 (4.60)
40.80 (5.40)
0.123
MCV(fL)
85.20 (6.30)
84.80 (6.80)
85.35 (8.70)
0.252
RDW (%)
13.50 (1.10)
13.50 (1.10)
13.30 (1.10)
0.691
Platelets (1,000 per count)
204 (86)
211 (49)
199 (100)
0.924
Recent WBC (1,000 per count)
8.50 (3.00)
8.40 (3.60)
8.50 (3.00)
0.894
Recent Hemoglobin (g/dL)
12.10 (2.90)
13.20 (2.45)
11.40 (2.40)
0.025
Recent Hematocrit (%)
37.30 (7.80)
39.10 (5.25)
34.40 (6.80)
0.017
Recent MCV(fL)
87.50 (5.60)
87.40 (7.30)
87.80 (5.40)
0.978
Recent RDW (%)
13.50 (1.65)
13.30 (2.20)
13.50 (1.60)
0.856
Recent Platelets (1,000 per count)
193 (90)
199 (92)
183 (90)
0.698
LVEDVI (ml/m2)
81.60 (19.20)
79.60 (23.65)
82.90 (22.60)
0.330
LVESVI (ml/m2)
34.66 (12.91)
35.23 (12.80)
34.10 (17.25)
0.919
LVSVI (ml/m2)
49.40 (16.00)
45.60 (13.05)
49.50 (21.80)
0.209
LVEF (%)
59 (13)
56 (11)
61 (14)
0.556
LVCO (L/min)
5.25 (2.40)
4.95 (0.50)
5.70 (2.75)
0.342
RVEDVI (ml/m2)
127.00 (44.20)
122.50 (43.60)
132.87 (36.5)
0.132
RVESVI (ml/m2)
66.80 (27.40)
63.05 (32.20)
67.35 (23.08)
0.452
RVSVI (ml/m2)
95.60 (56.28)
83.05 (44.67)
104.85 (56.25)
0.062
RVEF (%)
46.50 (14)
46.00 (15)
47.50 (12.5)
0.587
*Discrete variables are provided as frequency (%). Continuous variables are provided as mean ± standard deviation if normally distributed and median (interquartile range) if not.
PVR: Pulmonary Valve Replacement; WBC: White Blood Cell; MCV: Mean Corpuscular Volume; RDW: Red Blood Cell Distribution Width; LVEDVI: Left Ventricle End Diastolic Volume Index; LVESVI: Left Ventricle End Systolic Volume Index; LVSVI: Left Ventricle Stroke Volume Index; LVEF: Left Ventricle Ejection Fraction; LVCO: Left Ventricle Cardiac Output; RVEDVI: Right Ventricle End Diastolic Volume Index; RVESVI: Right Ventricle End Systolic Volume Index; RVSVI: Right Ventricle Stroke Volume Index; RVEF: Right Ventricle Ejection Fraction
Table 1: Characteristics of the study population overall and by replacement*.
These findings were confirmed in multivariable logistic regression analyses. The cMRI-RV measurements did not show any statistically significant association with PVR (RVEDVI OR 1.04, p=0.979; RVESVI OR 1.62, p=0.765; and RVEF OR 0.64, p=0.573 respectively). An RDW = 15% was associated with a twofold recourse to PVR (OR 2.05) but failed to reach statistical significance (p=0.559) (Table 2).
Outcome = Pulmonary Valve Replacement
Variable
OR (95%CI)
p
RDW=15%
2.05 (0.18-23.03)
0.560
RVEDVI>150 ml/m2
1.04 (0.05-22.13)
0.979
RVESVI>85 ml/m2
1.62 (0.07-38.67)
0.765
RVEF<45%
0.65 (0.14-3.00)
0.573
RDW: Red Blood Cell Distribution Width; RVEDVI: Right Ventricle End Diastolic Volume Index; RVESVI: Right
Ventricle End Systolic Volume Index; RVEF: Right Ventricle Ejection Fraction
Table 2: Multivariable logistic regression.
In further analyses, the AUC curve of high RDW at predicting PVR was 0.54. There were no statistically significant differences between the AUCs of high RDW and the RV measurements in predicting PVR (p=0.868) (Figure 1). Adding high RDW to a prediction model consisting of the 3 cMRI-RV variables resulted in a sizeable improvement of its C-statistic (from 0.55 to 0.59) though not statistically significant (p=0.641).
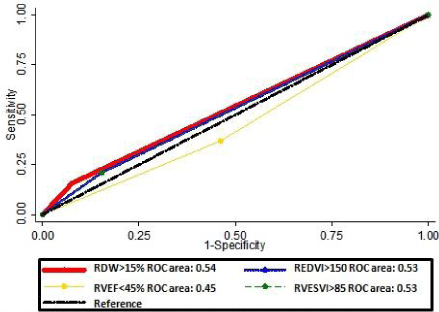
Figure 1: Areas under the receiver operating characteristics*.
RDW: Red Blood Cell Distribution Width; RVEDVI: Right Ventricle End
Diastolic Volume Index; RVESVI: Right Ventricle End Systolic Volume Index;
RVEF: Right Ventricle Ejection Fraction
Discussion
The timing of PVR in patients with rToF is an important topic of clinical research in ACHD. The gold standard to date has been cMRIbased RV measurements. RDW, an index in the variability of the size of circulating red cells, is routinely reported by automated laboratory equipment used to perform complete blood counts. Although its use had been generally limited to the evaluation of microcytic anemia, mounting evidence suggests additional roles. The present study in a sample population of adult rToF patients demonstrated a twofold association of an elevated RDW with recourse to PVR without reaching statistical significance. Of note, in this cohort of patients, RDW fared as well as cMRI-based RV measurements in predicting recourse to PVR as there was no statistically significant difference between their C-statistics. These results suggest that cMRI, currently considered the gold standard of RV functional assessment and a vital indicator for sequelae of pulmonic valve insufficiency may not stand as the optimal decision-making tool in determining management options for patients with rToF. Furthermore, patients who underwent PVR did not have any significant difference in their right ventricular volume and function as compared with the PVR-free group, suggesting that clinical impetus was a primary metric. This validates the effort behind exploring additional tools and perioperative markers to guide decision making in this challenging group of patients.
Evaluating these subjects for PVR is a top priority as they remain at high risk for atrial and ventricular arrhythmias or even sudden cardiac death. While most adults with ToF have a degree of hemodynamic dysfunction, these lesions can remain clinically silent for years. Replacement of the pulmonary valve becomes increasingly necessary with clinically significant symptoms or quantifiable signs of RV dysfunction and worsening pulmonary regurgitation on echocardiogram and cMRI [20]. Electrocardiograms have been previously analyzed in ToF patients demonstrating that QRS prolongation correlates with RV dilatation and may be predictive of PVR [21]. Biomarkers have been suggested as predictors of PVR in rToF adults. Hirono et al. proposed NT-pro-brain natriuretic peptide (BNP), a marker of congestive heart failure and myocyte stress, as a potential surrogate for cMRI [22]. A total of 58 patients were evaluated and the AUC for NT-pro-BNP for reoperation was found to be 0.950 (P<0.001). However, their analysis did not compare NT-pro-BNP to cMRI-based measurements with regard to recourse to PVR.
RDW, an independent predictor of mortality [18,19], has been studied as a biomarker for several pathologies. In the Candesartan in Heart Failure: assessment of reduction in mortality and morbidity (CHARM) cohort, RDW was found to be a strong independent predictor of morbidity and mortality in a sample population of 2,679 patients with chronic heart failure [5]. Similarly, the multicenter prospective Study of Anemia in a Heart Failure Population (STAMINA-HFP) registry showed that every 1% increase in RDW value was strongly associated with hospitalization, and mortality [6].
Other investigators have explored RDW as a marker for adverse events in a variety of conditions. Hampole et al. [7] found that RDW was an independent predictor of mortality in 162 patients with pulmonary hypertension followed prospectively over a two year period. In patients experiencing acute myocardial infarction (MI), a graded increase in mortality rates was seen as RDW increased throughout the hospital course [8]. In another study of 2,304 patients admitted to the hospital with chest pain, elevated RDW was predictive of acute MI [9]. A post hoc analysis of 4,111 participants of the Cholesterol and Recurrent Events (CARE) Trial found a graded relationship between RDW and risk of both adverse cardiovascular events and death in patients with a history of acute myocardial infarction [10]. In one of the largest studies to assess this relationship Zalawadiya et al. [11] analyzed data from 7,556 participants in the National Health and Nutrition Examination Surveys (NHANES) III with regard to the association between elevated RDW and coronary artery disease. This large cohort demonstrated that individuals with elevated RDW have greater odds of being in the intermediate risk 10- year Framingham category.
RDW has demonstrated its value in mortality prediction outside the acute setting. A study of 389 male patients presenting for cardiac catheterization found that elevated RDW was a strong and independent predictor of all cause mortality in patients without anemia [12]. A larger study by Poludasu et al. [13] with 859 patients followed for three years showed similar findings.
The value of RDW may encompass clinical decision making and healthcare cost. In a sample population of 503 patients admitted to the hospital for unstable angina or non-ST-elevation MI, RDW was predictive of recourse to surgical intervention (coronary artery bypass grafting) as well as hospital readmission outside of the 30-day period [14,15].
To the extent of our knowledge, only one investigation aside from the current one has evaluated RDW as a predictor of outcomes in patients with ToF. In a retrospective analysis of 94 patients who had undergone surgical corrective repair [16], Kumar et al. found that patients with elevated RDW had longer post-operative intensive care unit length of stays, longer total hospitalizations, longer times on mechanical ventilation, and more post-operative infections. Of note, this study was limited by its small sample size.
Although RDW is an emerging biomarker with cumulative literature about its association with prognosis and clinical decision making, its potential underlying biological mechanism remains unknown. One conceivable pathway is subclinical disease processes that cause a subtle dysregulation of erythrocyte homeostasis expressed in RDW. Another is chronic hypoxia which, in cyanotic patients, is known to stimulate erythropoeisis. As patients with repaired ToF age, worsening pulmonary valve regurgitation causes remodeling of the right ventricle with resultant congestive heart failure and poor oxygenation.
Several limitations should be considered in the interpretation of these results. The present study is a retrospective analysis and has all the inherent limitations of such a design. For example there was no consistent data on iron profile in the study subjects, an important factor in the interpretation of RDW measurements. This is mainly accounted for by the fact that the association was independent of anemia. As a single-institution investigation, the findings may reflect a specific standard of practice, thus limiting external validity. However, the study population’s clinical characteristics did not differ from those in other studies suggesting reasonable generalizability. The intervals for the measured effect sizes were large, reflective of the small sample size. This low statistical power may be behind the lack of statistical significance of the results and may be actually underestimating a true association. The baseline characteristics between the outcome groups were similar, but there were certain differences that were adjusted for. Despite accounting for hemoglobin and hematocrit and assessing for potential confounding factors and effect modifiers, residual confounding variables could lead to the observed results. As with all analyses of observational data, this study cannot distinguish causality from association. Finally, no proven mechanism for the potential association between RDW and the observed outcome is provided by the current analysis.
Conclusion
The present study shows no statistically significant difference between an elevated RDW and cMRI-based RV measurements in predicting recourse to PVR in adult rToF patients. The analysis was limited by its small sample size and retrospective design. These results are to be examined in the setting of a limited study population size that restricts the power of the analysis. However, as RDW is an inexpensive and readily available biomarker, it deserves further investigation in higher powered large multicenter datasets to fully determine its role as an additional predictive tool for PVR timing in adult rToF patients.
Acknowledgment
The authors would like to thank Scott Pilgrim, MD for his contribution to this project.
References
- Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009; 374: 1462-1471.
- Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009; 35: 156-164.
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions. JACC. 2008; 52: e143-e263.
- Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and allcause mortality in critically ill patients. Crit Care Med. 2011; 39:1913-1921.
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007; 50: 40-47.
- Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010; 16: 230-238.
- Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009; 104: 868-872.
- Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010; 105: 312-317.
- Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Franchini M, Guidi GC, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009; 47: 353-357.
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008; 117: 163-168.
- Zalawadiya SK, Veeranna V, Niraj A, Pradhan J, Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010; 106: 988-993.
- Cavusoglu E, Chopra V, Gupta A, Battala VR, Poludasu S, Eng C, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010; 141: 141-146.
- Poludasu S, Marmur JD, Weedon J, Khan W, Cavusoglu E. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2009; 102: 581-587.
- Ephrem G. Red blood cell distribution width is a predictor of readmission in cardiac patients. Clin Cardiol. 2013; 36: 293-299.
- Ephrem G, Kanei Y. Elevated red blood cell distribution width is associated with higher recourse to coronary artery bypass graft. Cardiology. 2012; 123: 135-141.
- Kumar S, Sudhakar A, Mohan M, Balachandran R, Raj B, Sumangala SG, et al. Elevated red cell distribution width is associated with delayed postoperative recovery after correction of Tetralogy of Fallot. Ann Pediatr Cardiol. 2013; 6: 121-125.
- Holmes KW. Timing of pulmonary valve replacement in tetralogy of fallot using cardiac magnetic resonance imaging: an evolving process. J Am Coll Cardiol. 2012; 60: 1015-1017.
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009; 169: 588-594.
- Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010; 65: 258-265.
- Oechslin EN, Harrison DA, Harris L, Downar E, Webb GD, Siu SS, et al. Reoperation in adults with repair of tetralogy of fallot: indications and outcomes. J Thorac Cardiovasc Surg. 1999; 118: 245-251.
- Therrien J, Siu SC, Harris L, Dore A, Niwa K, Janousek J, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001; 103: 2489-2494.
- Hirono K, Sekine M, Shiba N, Hayashi S, Nakaoka H, Ibuki K, et al. N-terminal pro-brain natriuretic peptide as a predictor of reoperation in children with surgically corrected tetralogy of fallot. Circ J. 2014; 78: 693-700.