
Special Article – Molecular Biomarkers
Austin Biomark Diagn. 2015; 2(2): 1019.
Kallistatin: A Novel Biomarker for Hypertension, Organ Injury and Cancer
Julie Chao*, Grant Bledsoe and Lee Chao
Department of Biochemistry and Molecular Biology, Medical University of South Carolina, USA
*Corresponding author: Julie Chao, Department of Biochemistry and Molecular Biology, Medical University of South Carolina, 173 Ashley Ave., Charleston, South Carolina, USA
Received: June 15, 2015; Accepted: September 01, 2015; Published: September 07, 2015
Abstract
Kallistatin has pleiotropic effects in vasodilation and inhibition of inflammation, angiogenesis, oxidative stress, fibrosis, and cancer progression. Kallistatin administration by gene or protein delivery is observed to offer protection against a large number of pathological conditions in animal models, such as hypertension, cardiovascular and organ damage, arthritis, sepsis, influenza virus infection, tumor growth and metastasis. However, injection of a neutralizing Kallistatin antibody into hypertensive rats aggravates cardiovascular and renal injury in association with increased inflammation, oxidative stress and tissue remodeling. Thus, animal studies show that kallistatin treatment exerts beneficial effects against hypertension, organ damage and cancer development. Moreover, serum kallistatin levels are markedly reduced in several animal models of hypertension and cardiac, cerebral and renal injury. Importantly, kallistatin levels in circulation, body fluids or tissues are significantly lower in patients with liver disease, septic syndrome, diabetic retinopathy, severe pneumonia, inflammatory bowel disease, and cancer of the colon and prostate. Furthermore, reduced plasma kallistatin levels are associated with adiposity and metabolic risk in apparently healthy African American youths. The focus of this review is to highlight circulating kallistatin as a potential new biomarker for human diseases.
Keywords: Kallistatin; Hypertension; Inflammation; Vascular injury; Organ damage; Infection; Sepsis; Cancer
Abbreviations
eNOS: endothelial Nitric Oxide Synthase; EPC: Endothelial Progenitor Cell; HMGB1: High Mobility Group Box-1; I/R: Ischemia/Reperfusion; KBP: Kallikrein-Binding Protein; LPS: Lipopolysaccharide; MI: Myocardial Infarction; NO: Nitric Oxide; ROS: Reactive Oxygen Species; SHR: Spontaneously Hypertensive Rat; SOCS3: Suppressor of Cytokine Signaling 3; SPTBN1: β II-Spectrin; STZ: Streptozotocin; TGF-β: Transforming Growth Factor-β; TLR4: Toll-Like Receptor 4; TNF-a: Tumor Necrosis Factor-a; VEGF: Vascular Endothelial Growth Factor
Introduction
Kallistatin is an endogenous protein that was first discovered in human plasma as a tissue Kallikrein-Binding Protein (KBP) [1]. Tissue kallikrein is a serine proteinase that releases vasodilating kinin peptides from kininogen substrate [2]. The tissue kallikrein-kinin system is involved in mediating beneficial effects in hypertension as well as cardiac, cerebral and renal injury [3]. KBP was later identified as a serine proteinase inhibitor (serpin) because of its ability to inhibit tissue kallikrein activity, and was subsequently named “kallistatin” [4-9]. Kallistatin is mainly expressed in the liver, but is also present in the heart, kidney and blood vessel [9-11]. Kallistatin protein contains two structural elements: an active site and a heparin-binding domain [12-14]. The active site of kallistatin is crucial for complex formation with tissue kallikrein, and thus tissue kallikrein inhibition [6]. Kallistatin sheparin-binding domain, however, is essential for antagonizing signaling pathways mediated by Vascular Endothelial Growth Factor (VEGF), Tumor Necrosis Factor (TNF)-a, High Mobility Group Box-1 (HMGB1), and Transforming Growth Factor (TGF)-β [15-18]. Through its structural elements, kallistatin is able to modulate a wide spectrum of biological activities, such as blood pressure, angiogenesis, inflammation, apoptosis, fibrosis, and cancer.
Reduced kallistatin levels are associated with hypertension
Kallistatin is expressed in tissues relevant to cardiovascular function, and has consequently been shown to have vasodilating properties [19]. Spontaneously Hypertensive Rats (SHR) display markedly reduced circulating kallistatin levels compared to normotensive rats, indicating that kallistatin may be involved in maintaining normal blood pressure [5,20]. In addition to SHR, salt- and surgically-induced hypertensive rats exhibit decreased serum kallistatin levels, as measured by specific Enzyme-Linked Immunosorbent Assay (ELISA) [21] (Figure 1). Kallistatin has been demonstrated to stimulate vasodilation, as an intravenous injection of purified kallistatin induced a rapid and transient reduction of blood pressure in both normotensive and hypertensive rats [19]. Vasorelaxation in isolated rataortic rings was also observed upon kallistatin administration [19]. Neither the blood pressure-lowering effect nor the vasorelaxation ability of kallistatin was abolished by icatibant (Hoe140, a kinin B2receptor antagonist), indicating that kallistatin-mediated vasodilation is unrelated to the tissue kallikreinkinin system [19]. Furthermore, over expression of rat kallistatin in transgenic mice resulted in significantly lower blood pressure as compared to control mice [22]. Likewise, gene delivery of human kallistatin caused a protracted blood pressure reduction in SHR [23]. These studies demonstrate that kallistatin is a novel vasodilating agent; and that circulating kallistatin levels are associated with the development of hypertension.
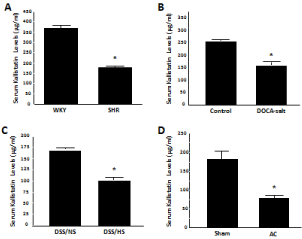
Figure 1: Serumkallistatin (rat KBP) levels are reduced in hypertensive rat
models. (A) Spontaneously Hypertensive Rats (SHR), (B) Deoxycorticosterone
Acetate (DOCA)-salt hypertensive rats, (C) Dahl Salt-Sensitive rats on a High
Salt diet (DSS/HS), and (D) hypertensive rats with Aortic Constriction (AC).
WKY: Wistar-Kyoto rats; DSS/NS: Dahl salt-sensitive rats on normal salt diet.
*P<0.001 vs. control group.
Decreased kallistatin levels during inflammation and organ injury
Kallistatin is considered to be a negative acute-phase protein. Circulating kallistatin as well ashepatic expression levels are rapidly reduced within 24 hours after Lipopolysaccharide (LPS)- induced endotoxemia in mice [24]. Serum kallistatin levels are also significantly lower in rat models with organ damage, such as Streptozotocin (STZ)-induced diabetes, gentamicin-induced renal toxicity, cardiac Ischemia/Reperfusion (I/R) and cerebral ischemic stroke, as measured by ELISA [21] (Figure 2). Similarly, circulating kallistatin levels are markedly decreased in patients with septic syndrome and liver disease [25]. However, kallistatin exhibits potent anti-inflammatory activity. For example, kallistatin gene delivery suppressed inflammatory responses and joint swelling in arthritic rats [26]. In addition, kallistatin administration into rat heart improved cardiac function and inhibited inflammatory cell infiltration after acute myocardial I/R and chronic Myocardial Infarction (MI) [27,28]. Kallistatin treatment also protected against renal injury, inflammation and oxidative stress in hypertensive rats [29,30]. Moreover, kallistatin gene transfer attenuated mortality, inflammation, and liver and skin damage in mice with streptococcal infection [31]. In cultured endothelial cells, kallistatin inhibited TNF-a-induced NF-κB activation and inflammatory gene expression [16]. Furthermore, kallistatin inhibited vascular inflammation by stimulating endothelial Nitric Oxide Synthase (eNOS) expression and activation, and thus NO formation, in endothelial and Endothelial Progenitor Cells (EPCs) [30,32,33]. Therefore, kallistatin is an antiinflammatory agent, and its administration inhibits inflammatory responses and organ damage.
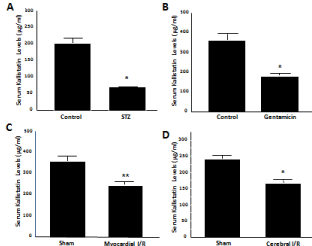
Figure 1: Serum kallistatin (rat KBP) levels are reduced innormotensive
rat models with oxidative organ damage. (A) Streptozotocin (STZ)-
induced diabetic rats, (B) gentamicin-induced nephrotoxic rats, (C) rats
with myocardial ischemia/reperfusion (I/R), and (D) rats with cerebral I/R.
*P<0.001 and **P<0.01 vs. control group.
Reduced kallistatin levels in cancer development
Kallistatin protective role in cancer progression has been documented in animal models and cultured cells [34-41]. Injection of the kallistatin gene into am urine model of pre-established breast cancer xenografts resulted in significant suppression of tumor growth and blood vessel numbers [34]. Systemic kallistatin gene delivery into mice markedly decreased tumor metastasis into lungs, which was accompanied by reduced angiogenesis and inflammation; kallistatin treatment also enhanced survival of tumor-bearing mice [41]. Moreover, up-regulation of kallistatin by SPTBN1 (β II-spectrin) reduced hepatocellular carcinoma progression in mice through suppression of Wnt signaling [42]. Kallistatin was also shown to inhibit Wnt-induced motility and invasion of cultured breast cancer cells through interaction with the Wnt co-receptor low-density Lipoprotein Receptor-related Protein 6 (LRP6) [43]. Additionally, kallistatin induced apoptotic cell death in human colorectal and breast cancer cells in vitro [40,44]. These findings indicate that kallistatin attenuates cancer development by inhibiting angiogenesis, inflammation, cancer cell growth, migration and invasion, and by inducing cancer cell apoptosis. Moreover, serum kallistatin levels are significantly decreased in patients with colon and prostate cancer, as measured by ELISA [25] (Figure 3). Thus, circulating kallistatin levels may serve as a predictive biomarker for cancer progression.
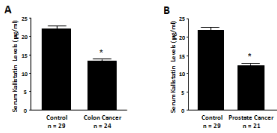
Figure 3: Serumkallistatin levels in are reduced in patients with (A) colon and
(B) prostate cancer.*P<0.05 vs. control group.
Kallistatin levels inversely correlate to oxidative stress
The pathogenesis of hypertension and cardiovascular and renal diseases is tightly linked to increased oxidative stress and reduced NO bioavailability [45]. Time-dependent elevation of circulating oxygen species are associated with reduced kallistatin levels in animal models of hypertension and cardiovascular and renal injury [29,46]. Stimulation of NO formation by kallistatin may lead to inhibition of oxidative stress and thus multi-organ damage. In rat models of myocardial I/R, MI, and hypertension, attenuation of organ injury by kallistatin gene transfer was accompanied by decreased ROS production and increased eNOS and NO levels [27-30]. Conversely, endogenous kallistatin depletion by neutralizing antibody increased oxidative stress and aggravated cardiovascular and renal damage in hypertensive rats [21]. Moreover, kallistatin through its anti-oxidant activity protected against CCl4-induced liver fibrosis in rats as well as LPS-induced acute lung injury in mice [47,48]. Furthermore, kallistatin reducedH2O2-mediated oxidative stress and NADPH oxidase expression in rat corneal epithelium and human hepatic Stellate cells [47,49]. NO has been shown to inhibit of NAD (P)H oxidase activity [50]; thus, stimulation of NO formation by kallistatin may lead to the attenuation of oxidative stress. Indeed, kallistatin was demonstrated to lower superoxide production and NAD(P)H oxidase activity provoked by TNF-a, H2O2, or angiotensin II via NO formation in cultured renal cells, cardiomyocytes, myofibroblasts, endothelial cells and EPCs [16,28-30,32,33]. Collectively, these studies show that kallistatin protects against the pathogenesis of hypertension and cardiovascular and renal diseases by anti-oxidant activity in conjunction with NO production. Therefore, an inverse relationship exists between kallistatin levels and oxidative stress.
Kallistatin is depleted in septic shock
A human kallistatin gene polymorphism was recently shown to correlate with a decreased risk of developing acute kidney injury during septic shock [51]. Kallistatin levels are markedly reduced in both humans and mice with sepsis syndrome [24,25]. However, kallistatin administration protects against lethality and organ injury in animal models of toxic septic shock. For example, transgenic mice expressing rat kallistatin are highly resistant to mortality induced by LPS [52]. Mice receiving kallistatin protein before polymicrobial sepsis exhibited attenuated lethality, peritoneal bacterial counts, splenic cell apoptosis, and renal injury and inflammation [17]. Furthermore, postponed kallistatin treatment after the onset of sepsis improved survival and prevented multi-organ injury in mouse models of established polymicrobialsepsis and endotoxemia [53]. Systemic inflammation was reduced by kallistatin as demonstrated by lower circulatory levels of TNF-a and HMGB1 [17,53]. Kallistatin up-regulated Suppressor of Cytokine Signaling 3 (SOCS3) expression in the kidney and lung, and decreased liver injury and hepatic TNF-a and Toll-Like Receptor 4 (TLR4) expression in septic mice [53]. These findings indicate that kallistatin is depleted during septic shock, yet exerts protective effects against multi-organ damage, inflammation and mortality in mice with sepsis syndrome.
Kallistatin levels are reduced in diabetes and obesity
Chronic inflammation of adipose tissue is related to the development of type 2 diabetes and obesity. Kallistatin levels are markedly reduced in the circulation and retinas of STZ-induced diabetic rats [54]. In addition, kallistatin levels are decreased in the vitreous fluid of patients with diabetic retinopathy; however, circulating kallistatin levels were shown to be elevated in patients with type 1 diabetes with vascular complications [55,56]. Moreover, kallistatin appears to play a potential role in cardio metabolic disorders, and perhaps the development of obesity, as plasma kallistatin levels are negatively correlated with waist circumference, low-density lipoprotein and total cholesterol, but positively associated with high-density lipoprotein in healthy, normotensive African American youths [57]. These findings indicate that reduced kallistatin levels are linked to diabetes and adiposity. It is known that TNF-a expression in infiltrating macrophages is elevated within adipose tissue of obese subjects, and thereby exerts autocrine and/ or paracrine effects [58]. TNF-a plays a key role in adipogenesis by maintaining active Wnt-signaling and inhibiting insulin-induced Akt-eNOS activation and NO formation, collectively leading to inflammation and insulin resistance [59]. Thus, kallistatin inhibits inflammation through multiple mechanisms: 1) antagonizing TNF- a-mediated cell signaling pathways [16]; 2) blocking HMGB1- mediatedinflammatory gene expression [17]; and 3) stimulating eNOS expression and activation and NO formation [30,32,33]. As a unique inhibitor of inflammation, kallistatin may play an important role in protection against the development of obesity and diabetes.
Kallistatin as anew biomarker for human diseases
Kallistatin levels in the circulation, body fluids or tissues are reduced in animal models of hypertension, cardiovascular, renal and cerebral injury, septic shock, diabetes, and hepatocellular carcinoma [5,24,29,42,46,52,54,60,61]. Moreover, kallistatin levels are decreased in patients with liver disease, septic shock, inflammatory bowel disease, severe pneumonia and acute respiratory distress syndrome [25,62,63]. Serum kallistatin levels are also significantly lower in patients with colon and prostate cancer (Figure 3). Likewise, plasma kallistatin levels are reduced in healthy African American youths with adiposity and cardio metabolic risk factors [57]. In addition, vitreal kallistatin levels are decreased in diabetic patients [55]. Therefore, kallistatin has the potential to be a molecular biomarker for patients with sepsis, cardiovascular and metabolic disorders, and cancer (Table 1).
Liver disease
Septic shock
Diabetic retinopathy
Inflammatory bowel disease
Obesity
Severe pneumonia
Acute respiratory distress syndrome
Colon and prostate cancer
Table 1: Kallistatin levels are reduced in human diseases.
Conclusion
Kallistatin is an endogenous protein that exerts a wide spectrum of biological activities that lead to protection against multi-organ damage and mortality. Kallistatin stimulates multiple pathways to inhibit angiogenesis, inflammation, apoptosis, oxidative stress, fibrosis and cancer. Animal studies have shown that circulating or tissue kallistatin levels are markedly reduced in hypertension, diabetes, cardiovascular and renal injury, whereas kallistatin administration reduces the pathogenesis of hypertension, organ dysfunction, inflammatory arthritis, sepsis, and cancer progression. Importantly, kallistatin levels are severely diminished in patients with liver disease, septic syndrome, diabetes, pneumonia, inflammatory bowel disease, and cancer, as well as African American adolescents with adiposity. Therefore, kallistatin may serve as a new biomarker for the prediction of patient outcomes.
Funding
This work was supported by the National Institutes of Health grants HL118516 and HL44083.
References
- Chao J, Tillman DM, Wang MY, Margolius HS, Chao L. Identification of a new tissue-kallikrein-binding protein. Biochem J. 1986; 239: 325-331.
- Erdos EG, Deddish PA. The kinin system: suggestions to broaden some prevailing concepts. Int Immunopharmacol. 2002; 2: 1741-1746.
- Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010; 391: 345-355.
- Chen LM, Chao L, Mayfield RK, Chao J. Differential interactions of human kallikrein-binding protein and alpha 1-antitrypsin with human tissue kallikrein. Biochem J. 1990; 267: 79-84.
- Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, et al. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem. 1990; 265: 16394-16401.
- Zhou GX, Chao L, Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J Biol Chem. 1992; 267: 25873-25880.
- Chai KX, Ward DC, Chao J, Chao L. Molecular cloning, sequence analysis, and chromosomal localization of the human protease inhibitor 4 (kallistatin) gene (PI4). Genomics. 1994; 23: 370-378.
- Chai KX, Chen LM, Chao J, Chao L. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem. 1993; 268: 24498-24505.
- Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. Biol Chem Hoppe Seyler. 1995; 376: 705-713.
- Chen LM, Song Q, Chao L, Chao J. Cellular localization of tissue kallikrein and kallistatin mRNAs in human kidney. Kidney Int. 1995; 48: 690-697.
- Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem. 1999; 47: 221-228.
- Chen VC, Chao L, Chao J. Reactive-site specificity of human kallistatin toward tissue kallikrein probed by site-directed mutagenesis. Biochim Biophys Acta. 2000; 1479: 237-246.
- Chen VC, Chao L, Chao J. Roles of the P1, P2, and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. J Biol Chem. 2000; 275: 38457-38466.
- Chen VC, Chao L, Pimenta DC, Bledsoe G, Juliano L, Chao J. Identification of a major heparin-binding site in kallistatin. J Biol Chem. 2001; 276: 1276-1284.
- Miao RQ, Chen V, Chao L, Chao J. Structural elements of kallistatin required for inhibition of angiogenesis. Am J Physiol Cell Physiol. 2003; 284: C1604-1613.
- Yin H, Gao L, Shen B, Chao L, Chao J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-alpha-induced nuclear factor kappaB activation. Hypertension. 2010; 56: 260-267.
- Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014; 142: 216-226.
- Guo Y, Li P, Bledsoe G, Yang ZR, Chao L, Chao J. Kallistatin inhibits TGF-β-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp Cell Res. 2015;.
- Chao J, Stallone JN, Liang YM, Chen LM, Wang DZ, Chao L. Kallistatin is a potent new vasodilator. J Clin Invest. 1997; 100: 11-17.
- Chao J, Chao L. A major difference of kallikrein-binding protein in spontaneously hypertensive versus normotensive rats. J Hypertens. 1988; 6: 551-557.
- Liu Y, Bledsoe G, Hagiwara M, Shen B, Chao L, Chao J. Depletion of endogenous kallistatin exacerbates renal and cardiovascular oxidative stress, inflammation, and organ remodeling. Am J Physiol Renal Physiol. 2012; 303: F1230-1238.
- Chen LM, Ma Jx, Liang YM, Chao L, Chao J. Tissue kallikrein-binding protein reduces blood pressure in transgenic mice. J Biol Chem. 1996; 271: 27590-27594.
- Chen LM, Chao L, Chao J. Adenovirus-mediated delivery of human kallistatin gene reduces blood pressure of spontaneously hypertensive rats. Hum Gene Ther. 1997; 8: 341-347.
- Chao J, Chen LM, Chai KX, Chao L. Expression of kallikrein-binding protein and alpha 1-antitrypsin genes in response to sex hormones, growth, inflammation and hypertension. Agents Actions Suppl. 1992; 38: 174-181.
- Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996; 127: 612-620.
- Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, et al. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005; 52: 1319-1324.
- Chao J, Yin H, Yao YY, Shen B, Smith RS Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006; 17: 1201-1213.
- Gao L, Yin H, S Smith R Jr, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008; 88: 1157-1166.
- Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008; 51: 1358-1365.
- Shen B, Gao L, Hsu YT, Bledsoe G, Hagiwara M, Chao L, et al. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am J Physiol Heart Circ Physiol. 2010; 299: H1419-1427.
- Lu SL, Tsai CY, Luo YH, Kuo CF, Lin WC, Chang YT, et al. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group A Streptococcal infection. Antimicrob Agents Chemother. 2013; 57: 5366-5372.
- Shen B, Smith RS Jr, Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J Biol Chem. 2009; 284: 35471-35478.
- Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, et al. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014; 3: e001194.
- Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002; 100: 3245-3252.
- Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu KW, et al. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol. 2007; 13: 4615-4619.
- Zhu B, Lu L, Cai W, Yang X, Li C, Yang Z, et al. Kallikrein-binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther. 2007; 6: 3297-3306.
- Lu L, Yang Z, Zhu B, Fang S, Yang X, Cai W, et al. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Lett. 2007; 257: 97-106.
- Jiang X, Li H, Qiao H, Jiang H, Xu R, Sun X. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci. 2009; 100: 2226-2233.
- Tse LY, Sun X, Jiang H, Dong X, Fung PW, Farzaneh F, et al. Adeno-associated virus-mediated expression of kallistatin suppresses local and remote hepatocellular carcinomas. J Gene Med. 2008; 10: 508-517.
- Yao Y, Li L, Huang X, Gu X, Xu Z, Zhang Y, et al. SERPINA3K induces apoptosis in human colorectal cancer cells via activating the Fas/FasL/caspase-8 signaling pathway. FEBS J. 2013; 280: 3244-3255.
- Shiau AL, Teo ML, Chen SY, Wang CR, Hsieh JL, Chang MY, et al. Inhibition of experimental lung metastasis by systemic lentiviral delivery of kallistatin. BMC Cancer. 2010; 10: 245.
- Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, et al. βII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015; 61: 598-612.
- Zhang J, Yang Z, Li P, Bledsoe G, Chao L, Chao J. Kallistatin antagonizes Wnt/β-catenin signaling and cancer cell motility via binding to low-density lipoprotein receptor-related protein 6. Mol Cell Biochem. 2013; 379: 295-301.
- Li P, Guo F, Bledsoe G, Yang ZR, Chao L, Chao J. Kallistatin induces breast cancer cell death by differential regulation of miR-21, miR-203 vs. miR-34a.
- Montezano AC, Touyz RM. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012; 28: 288-295.
- Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol. 2010; 298: H1048-1054.
- Huang X, Wang X, Lv Y, Xu L, Lin J, Diao Y. Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One. 2014; 9: e88498.
- Lin WC, Chen CW, Huang YW, Chao L, Chao J, Lin YS, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep. 2015; 5: 12463.
- Zhou T, Zong R, Zhang Z, Zhu C, Pan F, Xiao X, et al. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Invest Ophthalmol Vis Sci. 2012; 53: 5033-5043.
- Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997; 272: 32773-32778.
- Frank AJ, Sheu CC, Zhao Y, Chen F, Su L, Gong MN, et al. BCL2 genetic variants are associated with acute kidney injury in septic shock*. Crit Care Med. 2012; 40: 2116-2123.
- Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci. 1997; 60: 1431-1435.
- Li P, Guo Y, Bledsoe G, Yang ZR, Fan H, Chao L, et al. Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis. Crit Care. 2015; 19: 200.
- Hatcher HC, Ma JX, Chao J, Chao L, Ottlecz A. Kallikrein-binding protein levels are reduced in the retinas of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 1997; 38: 658-664.
- Ma JX, King LP, Yang Z, Crouch RK, Chao L, Chao J. Kallistatin in human ocular tissues: reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr Eye Res. 1996; 15: 1117-1123.
- Jenkins AJ, McBride JD, Januszewski AS, Karschimkus CS, Zhang B, O'Neal DN, et al. Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. J Angiogenes Res. 2010; 2: 19.
- Zhu H, Chao J, Kotak I, Guo D, Parikh SJ, Bhagatwala J, et al. Plasma kallistatin is associated with adiposity and cardiometabolic risk in apparently healthy African American adolescents. Metabolism. 2013; 62: 642-646.
- Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010; 11: 145-156.
- Hammarstedt A, Isakson P, Gustafson B, Smith U. Wnt-signaling is maintained and adipogenesis inhibited by TNFalpha but not MCP-1 and resistin. Biochem Biophys Res Commun. 2007; 357: 700-706.
- Chao C, Madeddu P, Wang C, Liang Y, Chao L, Chao J. Differential regulation of kallikrein, kininogen, and kallikrein-binding protein in arterial hypertensive rats. Am J Physiol. 1996; 271: F78-86.
- Luo Q, Siconolfi-Baez L, Annamaneni P, Bielawski MT, Novikoff PM, Angeletti RH. Altered protein expression at early-stage rat hepatic neoplasia. Am J Physiol Gastrointest Liver Physiol. 2007; 292: G1272-1282.
- Stadnicki A, Mazurek U, Plewka D, Wilczok T. Intestinal tissue kallikrein-kallistatin profile in inflammatory bowel disease. Int Immunopharmacol. 2003; 3: 939-944.
- Lin WC, Lu SL, Lin CF, Chen CW, Chao L, Chao J, et al. Plasma kallistatin levels in patients with severe community-acquired pneumonia. Crit Care. 2013; 17: R27.