Letter to the Editor
Austin J Biomed Eng. 2014;1(2): 1009.
Bactericidal and Therapeutic Effects of Pure Non-Pasteurized Honey on Mycobacterium avium Subspecies Paratuberculosis
Naser SA1*, Elwasila SM1, Alcedo K1, Riley II MK1 and Thanigachalam S1
1Burnett School of Biomedical Sciences, University of Central Florida, USA
*Corresponding author: :Saleh A Naser, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Building 20, BMS 136, 4110 Libra Drive, Orlando, FL 32816, USA.
Received: May 27, 2014; Accepted: May 29, 2014; Published: May 31, 2014
Letter to the Editor
Honey is an ancient remedy for various medical complications like pressure ulcers, wounds, and burns [1]. Despite the awareness of the medicinal benefits of honey, the literature is limited with information about the mechanism of action of honey or its interaction with microbes and host cells. Honey is produced in the salivary glands of Apismelliferadrone (male) bees [1]. Due to the different sources of nectar, various combinations of monosaccharide (glucose and fructose), disaccharide (maltose and sucrose), and oligosaccharide make up honey [2]. It is also rich in antioxidants, and contains hydrogen peroxide and nitric oxide; all of which aid in its antibacterial activity against a wide array of microorganisms [3].
Crohn’s diseaseis an inflammatory bowel disease, which is similar to Johne’s disease (also known as Paratuberculosis), and most likely caused by Mycobacterium subspecies paratuberculosis (MAP) as a possible etiological agent [4].Treatment of Crohn’s disease patients with anti-MAP therapy is encouraged to eradicate MAP and alleviate the patients from the chronic symptoms. This study aimed at investigating variety of honey solutions against MAP using in vitro microbiologic techniques in an attempt to provide Crohn’s patients with dietary anti-microbial supplements.
The study was designed to identify the bactericidal properties of common, non-pasteurized Orange Blossom Honey as they pertain to the survival of MAP. We used the 460 TB-BACTEC™ culture systems to monitor the antimicrobial activity of honey in a liquid environment against MAP. The BACTEC™ system provides a media that is suitable for the growth of MAP and also provides the investigator with an easy means of quantifying the growth of the bacteria (Growth Index indication of 0-10 indicating no survival and a level of 999 indicating infestation). BACTEC™ media was inoculated with various concentrations of honey ranged from 0% to 50% and a consistent dose of MAP strain 18. The effectiveness of honey against MAP was also evaluated using non-pasteurized honey and autoclaved honey. Moreover, Honey effect against rapid grower microorganisms such as Escherichia coli and Methicillin Resistant Staphylococcus Aureus (MRSA) was demonstrated using nutrient agar plates. Zone of inhibition following culture incubation was sought and measured.
Honey
Concentration
GI
% Inhibition
Non –pasteurized
4.5%
377
60%
Autoclaved
4.5%
999
0%
Non-pasteurized
12.5%
72
95%
Autoclaved
12.5%
999
0%
Table 1: Determining antibacterial effects of autoclaved and non-pasteurized Orange blossom honey bee.
As shown in Table 1, Bactec cultures with no honey solutions showed a maximum growth index of 999 following weeks of incubation at 37oC. However, there was a 95% growth inhibition in Bactec cultures supplemented with 12.5% (w/v) non-pasteurized honey compared to 60% growth inhibition in those supplemented with 4.5% (w/v) non-pasteurized honey. In contrast, autoclaved honey showed no growth inhibition against MAP. Higher concentrations of non-pasteurized honey demonstrated no MAP growth survival. The fact that higher concentrations of autoclaved honey had no negative effect on MAP growth indicates that anti-MAP active ingredients are heat labile. We propose that the mechanism of action for honey’s anti-MAP activity may be attributed to the activity of honey’s glucose oxidase which leads to production of hydrogen peroxide. This confirms earlier observation where glucose oxidase activity was lost after heating honey at 250°F for 10 minutes [5]. Moreover, a higher sugar concentration may have an additive antibacterial effect[1] due to excessive osmotic pressure.
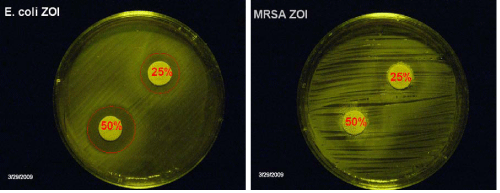
Non-pasteurized honey was also tested against E. coli and MRSA using the disk impregnation standard method. As shown in Figure (1A), the disk with the higher honey concentration (50% w/v) had a larger, distinctive ring of Zone of Inhibition (ZOI) compared to the lesser honey concentration (25% w/v), attributing its antimicrobial potency to excessive osmotic pressure. Although Figure (1B) also shows a larger ring of ZOI for 50% (w/v) honey compared to 25% (w/v), it is not as distinct. This indicates that it is not just osmotic pressure that prevents microbial growth, but H2O2 production by the enzyme glucose oxidase may also play a role. In Figure 1B, the catalase activity of MRSA breaks down hydrogen peroxide [6], thus preventing complete inhibition. As shown in Table 2, the anti-MAP activity in honey was bactericidal. MAP growth was inhibited by a higher concentration of honey. After two weeks of incubation, 25% (w/v) honey had a cidal effect against MAP. This was supported by the lack of growth when a sample of the honey-treated MAP culture was washed and inoculated into fresh media.
Figure 1: Disk impregnation method of testing antibacterial activity of honey. Zone of inhibition of (A) E. coli and (B) methicillin-resistant Staphylococcusaureus (MRSA).Honey was tested at 25% and 50% dilution in sterile water on nutrient agar plates. Agar plates were incubated at 37°Cfor 48 hours.
Concentration of Orange Blossom Honey
GI
% Inhibition
0%
999
0%
25%
13
~99%
Table 2: Determination of bactericidal activity of honey.MAP cells were harvested from Bactec culture media incubated with MAP and 25% honey following weeks of incubation. MAP cells were harvested, washed and then inoculated into fresh Bactec culture media without honey. Growth Index readings were recorded and Growth inhibition were calculated.
Honey was also tested in conjunction with antibiotics used against MAP. Interestingly enough, it appears as though the honey and the antibiotic cocktail had negated each other through an unknown mechanism. The implications of this process are of concern in terms of treatment for Crohn’s patients.
Throughout this study, honey has proven to have antibacterial effects against three different microorganisms: E.coli, MRSA, and MAP. Its high osmotic concentration and H2O2 production have been highlighted in this study as key players to its inhibitory activity. However, these are just two of the many intrinsic and extrinsic factors of honey that may play a role. Further study with a larger number of samples is encouraged to support these findings, as well as, testing honey from various different geological locations to compare the different antimicrobial activities they may have and to determine other factors that may be used against MAP in Crohn’s disease patients. Additionally, studies into Colony Collapse Disorder (CCD) and the effect on honey production are also necessary [7]. The causative agent of CCD is believed to be pesticides, so it’s possible that honey could contain pesticide residues which can contribute to the microbial inhibitory effect of honey.
References
- Yaghoobi R, Kazerouni A, Kazerouni O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J Nat Pharm Prod. 2013; 8: 100-104.
- Wang CW, Chen WT, Chang HT. Quantification of Saccharides in Honey Samples Through Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Using HgTe Nanostructures. J Am Soc Mass Spectrom, 2014.
- Al-Waili NS, Salom K, Butler G, Al Ghamdi AA. Honey and microbial infections: a review supporting the use of honey for microbial control. J Med Food. 2011; 14: 1079-1096.
- Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004; 364: 1039-1044.
- Chen C, Campbell LT, Blair SE, Carter DA. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front Microbiol. 2012; 3: 265.
- Grüner BM, Han SR, Meyer HG, Wulf U, Bhakdi S, Siegel EK. Characterization of a catalase-negative methicillin-resistant Staphylococcus aureus strain. J Clin Microbiol. 2007; 45: 2684-2685.
- Vanengelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. 2010; 103 Suppl 1: S80-95.